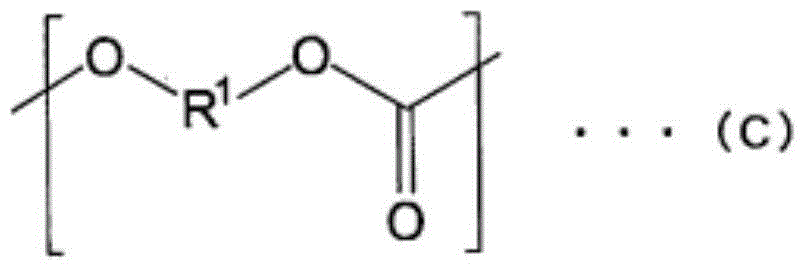
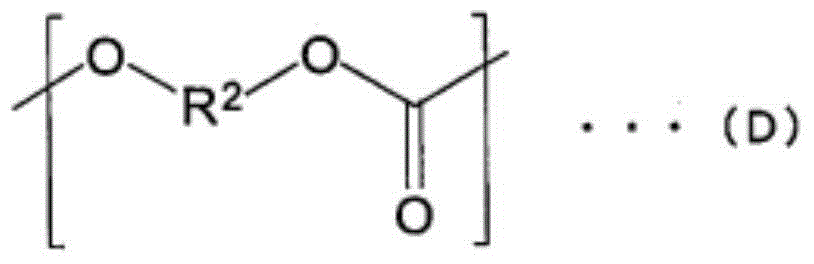
Polycarbonate diol and polyurethane using same
A technology of polycarbonate and polyurethane, applied in the field of polyurethane, can solve the problems of hard texture, limitation, poor chemical resistance, etc., and achieve the effect of excellent elastic recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0494] Examples and comparative examples are given below to explain the present invention more specifically, but as long as it does not exceed the gist of the present invention, it is not limited to these examples.
[0495] Hereinafter, the evaluation method of each physical property value is as follows.
[0496] [Evaluation method: polycarbonate diol]
[0497]
[0498] Dissolve polycarbonate diol in CDCl 3 , Measure 400MHz 1 H-NMR (AL-400 manufactured by JEOL Co., Ltd.) identifies phenoxy groups, dihydroxy compounds, and phenols based on the signal position of each component, and calculates the respective contents based on the integral value. At this time, the detection limit is 100 ppm as the weight of phenol relative to the weight of the entire sample, and dihydroxy compounds such as the compound represented by the above formula (A) and the compound represented by the above formula (B) are 0.1% by weight. In addition, the ratio of the phenoxy group was determined from the ratio o...
Embodiment 1-1
[0561]
[0562] In a 5L glass separable flask equipped with a stirrer, a distillate collector, and a pressure regulator, 1,4-butanediol (1,4BD): 768.5g, 1,10- Decanediol (1, 10DD): 768.5 g, diphenyl carbonate: 2563.0 g, magnesium acetate tetrahydrate aqueous solution: 6.6 mL (concentration: 8.4 g / L, magnesium acetate tetrahydrate: 55 mg), and replaced with nitrogen. Under stirring, the internal temperature was raised to 160°C, and the contents were dissolved by heating. Then, after the pressure was reduced to 24 kPa over 2 minutes, the phenol was removed from the system and allowed to react for 90 minutes. Next, the pressure was reduced to 9.3 kPa over 90 minutes, and further to 0.7 kPa over 30 minutes to continue the reaction, and then the temperature was raised to 170°C to remove the phenol and unreacted dihydroxy compound out of the system while reacting for 60 minutes. A composition containing polycarbonate diol is obtained.
[0563] The obtained polycarbonate diol-containi...
Embodiment 1-2~ Embodiment 1-13
[0572]
[0573] In the production of the polycarbonate diol of Example 1-1, all were carried out under the same conditions and methods except that the type and feed amount of the PCD polymerization raw materials were changed to those described in Table 1 The reaction yields a composition containing polycarbonate.
[0574] The obtained polycarbonate diol-containing composition was subjected to thin-film distillation in the same manner as in Example 1-1. Table 1 shows the evaluation results of properties and physical properties of the polycarbonate diol obtained by thin-film distillation.
[0575]
[0576] In the production of the polyurethane of Example 1-1, except that the type of polycarbonate diol (PCD) used and the feed amount of each raw material were changed to the PCD and the feed amount described in Table 3, all were the same. The conditions and methods are reacted to obtain a polyurethane solution. The properties and physical properties of the polyurethane are shown in Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com