A kind of boron series compound branched carboxylated nitrile rubber and its preparation method and application
A technology of carboxylated nitrile rubber and boric acid compound, which is applied in the field of boron compound branched carboxylated nitrile rubber and its preparation, can solve the problems of electrode polarization, low conductivity of solid polymer electrolyte, low room temperature ionic conductivity, etc. Achieve the effect of avoiding electrode polarization, avoiding high ion migration number, and high conductivity at room temperature or high temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
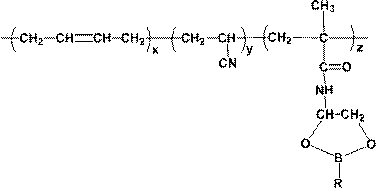
[0050] A kind of boron series compound branched carboxyl nitrile rubber of the present embodiment, its chemical structural formula is as shown in formula I:
[0051]
[0052] Formula I;
[0053] in:
[0054] x, y and z in formula I are all integers greater than zero;
[0055] R in formula I represents p-trifluoromethylphenyl ( ).
[0056] The preparation method of above-mentioned boron series compound branched carboxylated nitrile rubber, it may further comprise the steps:
[0057] Step 1, dissolve carboxylated nitrile rubber (XNBR) in tetrahydrofuran, stir at room temperature for 1.5 hours, after the carboxylated nitrile rubber is completely dissolved, the first mixture is obtained; in this example, the mass ratio of carboxylated nitrile rubber to tetrahydrofuran 1:1;
[0058] Step 2, dissolve 3-amino-1,2-propanediol (TriAN) and p-trifluoromethylphenylboronic acid (TriB) in tetrahydrofuran, then add dicyclohexylcarbodiimide and 4-dimethylaminopyridine, room temperature...
Embodiment 2
[0079] A kind of boron series compound branched carboxyl nitrile rubber of the present embodiment, its chemical structural formula is as shown in formula I:
[0080]
[0081] Formula I;
[0082] in:
[0083] x, y and z in formula I are all integers greater than zero;
[0084] R in formula I represents phenyl.
[0085] The preparation method of above-mentioned boron series compound branched carboxylated nitrile rubber, it may further comprise the steps:
[0086] Step 1, carboxylated nitrile butadiene rubber was dissolved in butanone, stirred at room temperature for 2 hours, after carboxylated nitrile butadiene rubber was completely dissolved, the first mixture was obtained; in the present embodiment, the mass ratio of carboxylated nitrile butadiene rubber to butanone was 1:2;
[0087] Step 2: Dissolve 3-amino-1,2-propanediol and phenylboronic acid in butanone, then add dicyclohexylcarbodiimide and 4-dimethylaminopyridine, and stir at room temperature for 8 hours to obtai...
Embodiment 3
[0099] A kind of boron series compound branched carboxyl nitrile rubber of the present embodiment, its chemical structural formula is as shown in formula I:
[0100]
[0101] Formula I;
[0102] in:
[0103] x, y and z in formula I are all integers greater than zero;
[0104] R in formula I represents pentafluorophenyl ( ).
[0105] The preparation method of above-mentioned boron series compound branched carboxylated nitrile rubber, it may further comprise the steps:
[0106] Step 1, carboxylated nitrile butadiene rubber is dissolved in tetrahydrofuran (THF), stirred at room temperature for 1 hour, after carboxylated nitrile butadiene rubber is completely dissolved, the first mixture is obtained; in the present embodiment, the mass ratio of carboxylated nitrile butadiene rubber to tetrahydrofuran is 1: 3;
[0107]Step 2, dissolving 3-amino-1,2-propanediol and the monosubstituted boronic acid compound represented by formula F in tetrahydrofuran, then adding dicyclohexy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com