Polycyclic amine modified (branched) polyether acrylate and preparation method thereof
A polyether acrylate and branched polyether technology, used in inks, additive processing, household appliances, etc., can solve the problems of low mechanical strength of cured films, large curing shrinkage, application and promotion restrictions, etc., and reduce amine migration. The effect of high stability, small curing shrinkage, and simple product purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
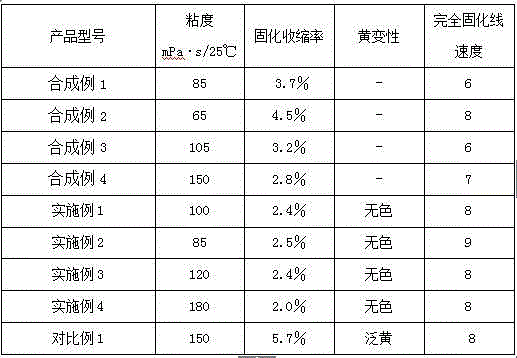
Synthetic example 1
[0049] Synthesis Example 1 Polyether Acrylate 1 (Mn≈862, functionality 3)
[0050] A kind of polyether triacrylate and preparation method thereof, comprises following two steps:
[0051] a. Transesterification reaction: Take 400g polyether triol (Mn≈700, Dow VOANOL 2070), 343.2g ethyl acrylate, 1.2g trinonylphenyl phosphite, 0.2g 2,6-di-tert-butyl Base-4-methylphenol, after uniform dispersion, add 5g of immobilized enzyme Novozyme 435 and 0.1g of lanthanum dodecylsulfonate, stir and react at 40°C for 24h;
[0052] b. Purification treatment: After filtering the mixture obtained in the previous step, the filtrate was subjected to vacuum rotary evaporation, and the ethanol and excess methacrylic acid generated by the reaction were removed to obtain 490.2 g of transparent and colorless esterified product (solid content 99.2%, viscosity 85 mPa ·s / 25℃, the esterification rate (based on polyether polyol as the base substrate, the same below) is 97.4%).
Synthetic example 2
[0053] Synthesis Example 2 Branched Polyether Acrylate 2 (Mn≈1022, Functionality 9)
[0054] A kind of branched polyether acrylate and preparation method thereof, concrete steps are as follows:
[0055] (1) Synthesis of branched polyether polyols
[0056] Glycerol is used as raw material to form branched polyether polyols (Mn≈536, functionality 9, branching degree 0.72) through one-step condensation under acidic conditions;
[0057] (2) Enzyme-catalyzed transesterification reaction, including the following two steps:
[0058] a. Transesterification reaction: take 300g of the above-mentioned branched polyether polyol, 550.0g methyl acrylate, 1.8g trinonylphenyl phosphite, 0.25g p-hydroxyanisole, after dispersing evenly, add 8g of curing enzyme Novozyme 435 and 0.1g lanthanum dodecylsulfonate, stirred and reacted at 50°C for 24h; the chemical reaction formula is as follows
[0059]
[0060] b. Purification treatment: After filtering the mixture obtained in the previous s...
Synthetic example 3
[0061] Synthesis Example 3 Polyether Acrylate 3 (Mn≈1162, functionality 3)
[0062] A kind of polyether triacrylate and preparation method thereof, comprises following two steps:
[0063] a. Transesterification reaction: Take 400g polyether triol (Mn≈1000, VOANOL CP-1055), 384.4g methyl acrylate, 3.2g trinonylphenyl phosphite, 0.4g p-hydroxyanisole, and disperse After uniformity, add 6g immobilized enzyme LVK-F100 and 0.1g lanthanum methanesulfonate, and stir and react at 40°C for 24h;
[0064] a. Purification treatment: After filtering the mixture obtained in the previous step, the filtrate was subjected to vacuum rotary evaporation, and the methanol and excess methyl acrylate generated by the reaction were removed to obtain 463.0 g of transparent and colorless esterified product (solid content 99.5%, viscosity 105 mPa· s / 25℃, the esterification rate is 97.1%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| Functional group degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com