Optimized cell culture medium and cell culture method, and application of optimized cell culture medium and cell culture method to preparation of protein and antibody
A medium and basic medium technology, applied in artificial cell constructs, cells modified by introducing foreign genetic material, animal cells, etc., can solve the problems of easy depletion of glutamine and accumulation of ammonium ions, and achieve high sugar The effect of the level of methylation and biological activity, simple production process, and stable protein properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1. Preparation of culture medium
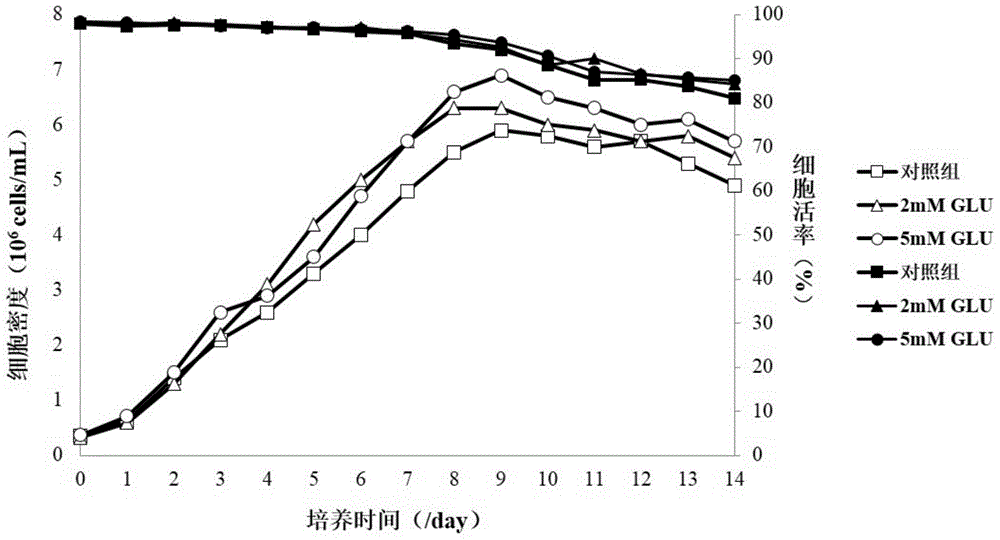
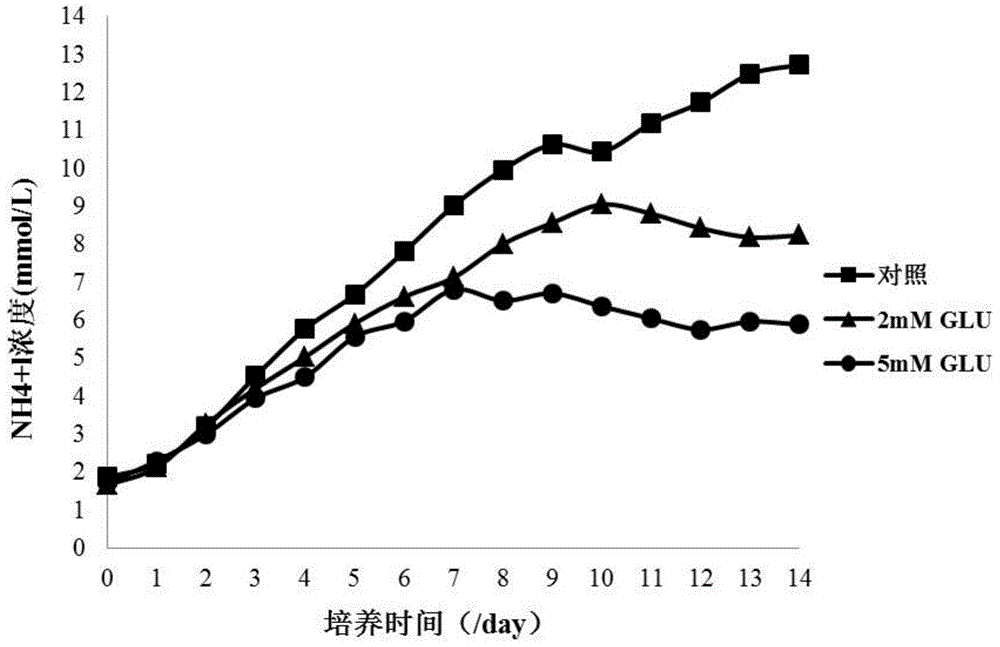
[0060] According to the formulations in Table 1 and Table 2, glutamic acid and 4mM glutamine of different concentrations were added to the basal medium as an optimized production medium in the culture process of recombinant CHO engineered cell lines, and only supplemented with the basal medium The comparison of glutamine control medium is used for the production and preparation of therapeutic target proteins under the same cell culture process conditions, so as to evaluate the effects of the two on the ammonium ion level of metabolic by-products, cell density, cell viability, and target protein glycosyl The influence of chemical level and biological activity, etc.
[0061] Table 1
[0062] Medium number
Addition 1
Addition 2
A
optimized medium
EX-CELL302
Glutamate 2mM
Glutamine 4mM
B
control medium
EX-CELL302
Glutamine 4mM
C
o...
Embodiment 2
[0068] Example 2 Recombinant CHO Engineering Cell Culture Expressing Recombinant Human Type II Tumor Necrosis Factor Receptor-Antibody (rhTNFRII-Fc) Fusion Protein
[0069] 1. Construction of rhTNFRII-Fc fusion protein expression vector
[0070] The dhfr (dihydrofolate reductase) expression unit in the pSV2-dhfr vector (ATCC product) was cloned into the pCDNA3.1(+) vector (Invitrogen company product) by PCR technology and DNA recombination technology to construct a mammal expressing DHFR Animal cell expression vector pBF01.
[0071] According to the gene sequence of hTNFRII (Genebank Accession No. AH006638) and human IgG1 Fc fragment gene sequence (WO9411026), primers were designed for PCR amplification and cloned into the pUC18 plasmid.
[0072] The primers were redesigned, and the cDNA fragments of hTNFRII and human IgG1 Fc obtained above were overlapped by PCR amplification to obtain the full-length cDNA fragment of the rhTNFRII-Fc fusion protein, which was cloned into the...
Embodiment 3
[0086] Example 3. Preparation of recombinant human type II tumor necrosis factor receptor-antibody (rhTNFRII-Fc) fusion protein
[0087] 1. Separation and purification of rhTNFRII-Fc fusion protein:
[0088] The cell culture fluid obtained in Example 2 was sequentially filtered through DOHC and B1HC depth filters of Millipore Company, and the filtrate was collected and purified by Protein A affinity chromatography.
[0089] After the MabselectSuRe (GE Company) chromatographic column was washed and balanced with PBS buffer, the cell culture supernatant was loaded at a speed of 50-300 cm / h, and washed with PBS buffer until all unbound proteins were eluted (with A280 was monitored), then the fusion protein was eluted with 20mmol / L citric acid (pH3.5), and the fusion protein stock solution was collected to adjust the pH value to 7.2 with Tris.
[0090] 2. Detection and analysis
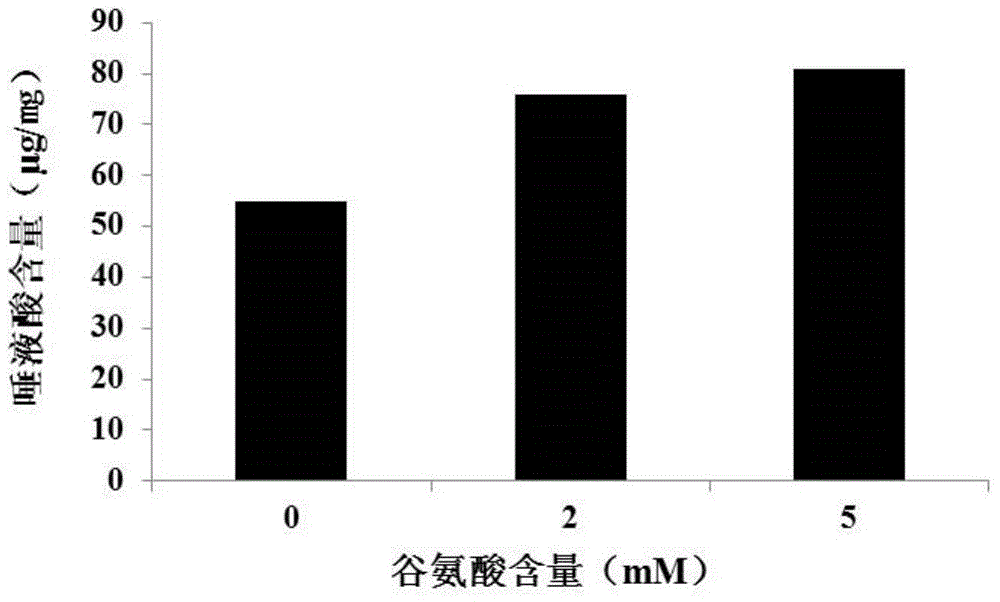
[0091] (1) Determination of sialic acid content in rhTNFRII-Fc fusion protein
[0092] According to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com