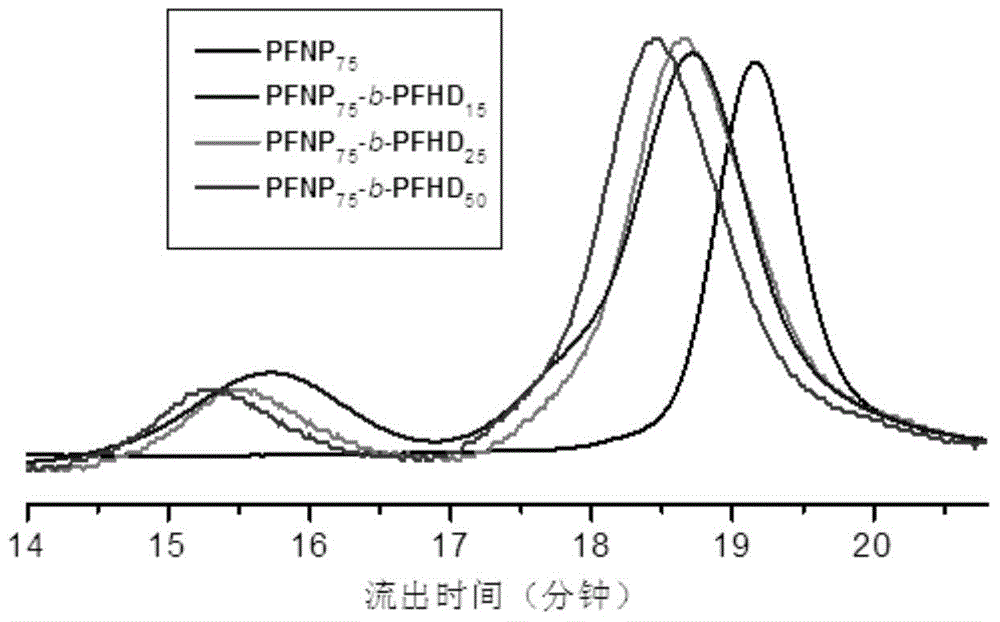
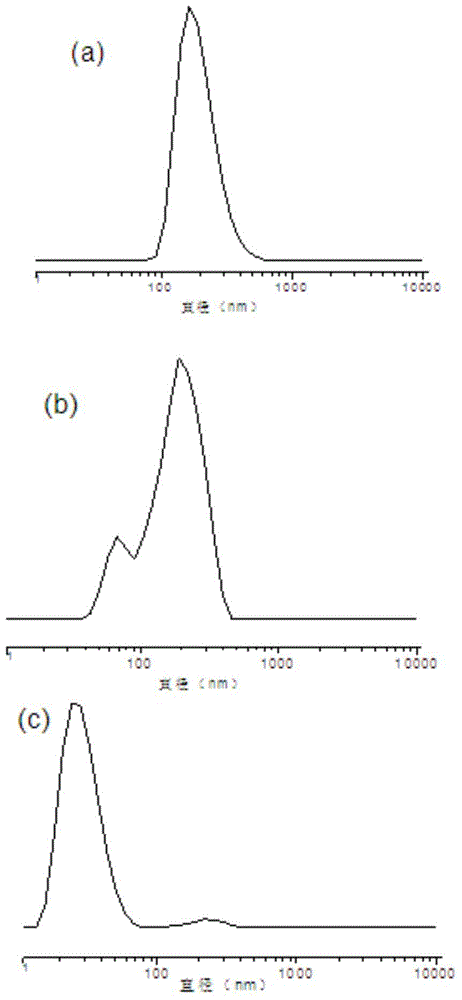
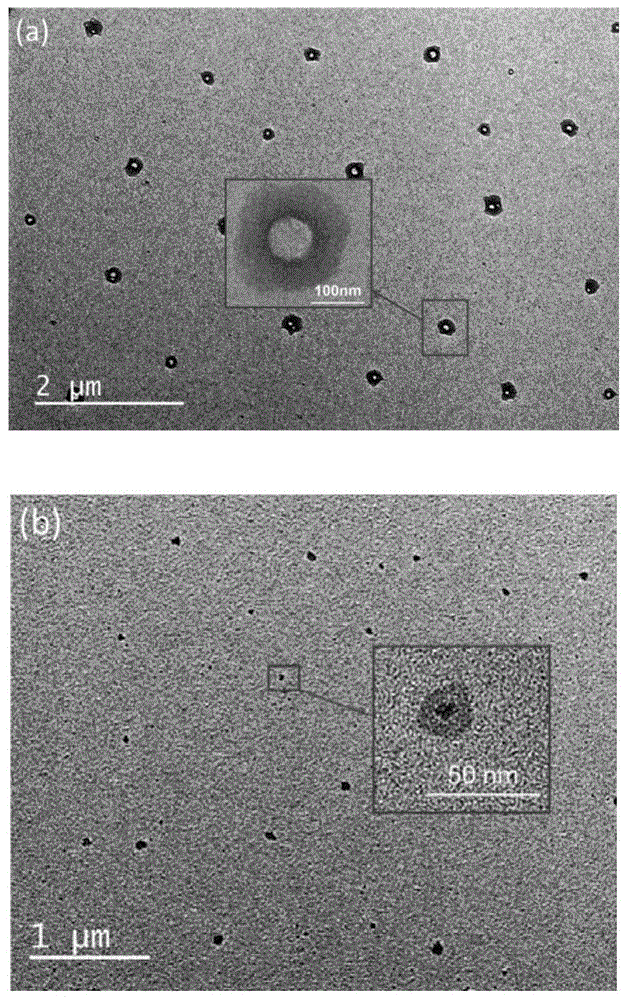
Fluorine-containing high-dielectricity polynorbornene-poly(1,6-heptadiyne) block copolymer and preparation method thereof
A technology of polynorbornene and block copolymers, which is applied in the field of high dielectric polymer preparation, and can solve problems related to dielectric materials that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Synthesis of N-3,5-difluorophenyl norbornene pyrrolidine
[0060] At room temperature, 3,6-endomethine-1,2,3,6-tetrahydrophthalic anhydride (9.84g, 60mmol) and 3,5-difluoroaniline (6.45g, 50mmol) were dissolved in 50mL in anhydrous 1,2-dichloroethane. Nitrogen was blown, and 0.3 equivalents of DMAP (1.83 g, 15 mmol) was added to the reaction solution. Reflux at 90°C for 36h, and the reaction ends. After several times of washing, drying, and solvent removal, the crude product was recrystallized to obtain white crystals, which were norbornene imide compounds containing 3,5-difluorophenyl. Under an ice bath, nitrogen gas was introduced into the reaction bottle, lithium aluminum hydride (4.56 g, 120 mmol) was quickly added into the bottle, and 100 mL of anhydrous ether was injected to obtain a suspension of lithium aluminum hydride. Subsequently, the norbornene imide compound (6.7 g, 24 mmol) containing 3,5-difluorophenyl was dissolved in 80 mL of dry dichloro...
Embodiment 2
[0062] Example 2: Synthesis of 4-pentafluorophenyl formate-1,6-heptadiyne
[0063]At room temperature, add 4-carboxy-1.6-heptadiyne (3.2g, 20mmol), pentafluorophenol (2.2g, 24mmol), DMAP (0.5g, 4mmol) and 60mL dichloromethane into the three-necked flask, stir and dissolve , EDCI (4.6g, 24mmol) was added to the system, stirring was continued for 36h, and the system changed from cloudy to clear. After the completion of the reaction was detected by thin-layer chromatography, it was washed with distilled water (30×6 mL) and saturated brine (30×3 mL) successively, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation to obtain a light yellow solid. Recrystallization was carried out with a mixed solvent of ethyl acetate and petroleum ether to obtain 4.3 g of white crystals (yield: 64%).
[0064] M.p.48.5-49.4℃. 1 H NMR (500MHz, CDCl 3 ,ppm): δ3.18(m,1H,OCOCH),2.83-2.81(m,4H,CHCCH 2 ),2.14(t,2H,CHCCH 2 ); 13 C NMR (CDCl 3 ): δ (ppm) 168.51 (CHC...
Embodiment 3
[0065] Example 3: Preparation of polynorbornene-poly(1,6-heptadiyne) block copolymers with different block ratios
[0066] In a 25mL Schlenk reaction tube, add N-3,5-difluorophenylnorbornene pyrrolidine (185.2mg, 0.75mmol), vacuumize and replace with high-purity nitrogen three times, add 2.7mL tetrahydrofuran, make it completely dissolved. In another 25mL Schlenk reaction tube, add 4-pentafluorophenyl formate-1,6-heptadiyne (45.2mg, 0.15mmol), vacuumize and replace with high-purity nitrogen three times, add 0.3mL tetrahydrofuran, make it completely dissolved. Under a nitrogen atmosphere, weigh Grubbs third-generation catalyst (8.8 mg, 0.01 mmol) into a third Schlenk tube, vacuumize and replace with nitrogen three times, and then add 1 mL of tetrahydrofuran. The reaction system was frozen with liquid nitrogen, vacuumed and filled with nitrogen, and then thawed after repeating the cycle 3 times; after preheating at 30°C for 10 minutes, the catalyst solution was quickly added t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com