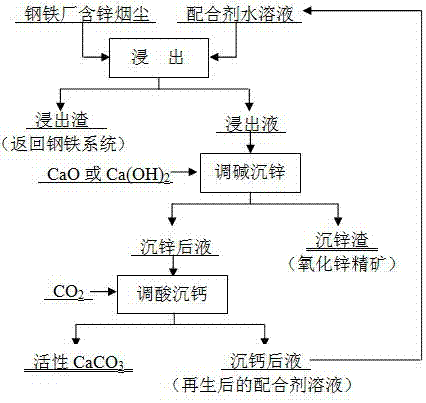
Method for enriching zinc oxide concentrate by wet treatment of zinc-containing dust in iron and steel plant
A technology of wet treatment and iron and steel plant, applied in the field of wet treatment of zinc-containing fumes in iron and steel plants, it can solve the problems of great harm to the human body and the environment, the lack of deep zinc removal, and the consumption of sulfuric acid in the leaching agent, etc., to achieve human and environmental toxicity Small side effects, low equipment anti-corrosion requirements, and low processing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Smoke dust from a steel factory, its main components (wt%): Zn 6.35, Fe 31.63, CaO 5.12, MgO 3.84, Cd1.20 and Pb 0.95; Reagent: Food grade glycine, content ≥ 99%; Industrial grade sodium glycine, Content ≥ 98%; analytically pure calcium oxide (CaO), content ≥ 98%; industrial carbon dioxide gas, content ≥ 99%.
[0038] (1) Leaching: At 75°C, use sodium glycine and glycine to make the pH value 8.5, and the total concentration of glycine is 1.2mol L -1 400mL aqueous solution of compounding agent, according to the liquid-solid ratio of 8:1, add 50g of smoke and dust for leaching, after reacting for 5h, separate the solid and liquid to obtain 335mL of leachate; among them, the concentration of Zn is 9.04g L -1 , Pb concentration 1.38g L -1 , Cd concentration 1.66g L -1 , Fe concentration 25.50mg·L -1 The leaching rate of Zn is 95.4%, the leaching rate of Pb is 91.0%, the leaching rate of Cd is 92.7%, and the leaching rate of Fe is 0.05%.
[0039] (2) Alkali adjustment and...
Embodiment 2
[0042] Smoke dust from a steel factory, its main components (wt%): Zn 10.37, Fe 23.32, CaO 7.63, MgO 1.07, Cd2.58 and Pb 1.09; Reagent: Food grade glycine, content ≥ 99%; Analytical pure sodium hydroxide, Content ≥ 99%; analytically pure calcium oxide (CaO), content ≥ 98%; industrial carbon dioxide gas, content ≥ 99%.
[0043] (1) Leaching: at 70°C, use glycine and sodium hydroxide to make the pH value 9, and the total concentration of glycine is 1.5mol L -1 300mL aqueous solution, according to the liquid-solid ratio of 6:1, add 50g of dust for leaching, react for 4h, separate the solid and liquid, and obtain 275mL of leaching solution; among them, the concentration of Zn is 17.80g L -1 , Pb concentration 1.84g L -1 , Cd concentration 4.30g L -1 , Fe concentration 35.60mg·L -1 ; The Zn leaching rate was 94.4%, the Pb leaching rate was 92.8%, the Cd leaching rate was 91.7%, and the Fe leaching rate was 0.08%. The leaching solution enters the next step of alkali adjustment a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com