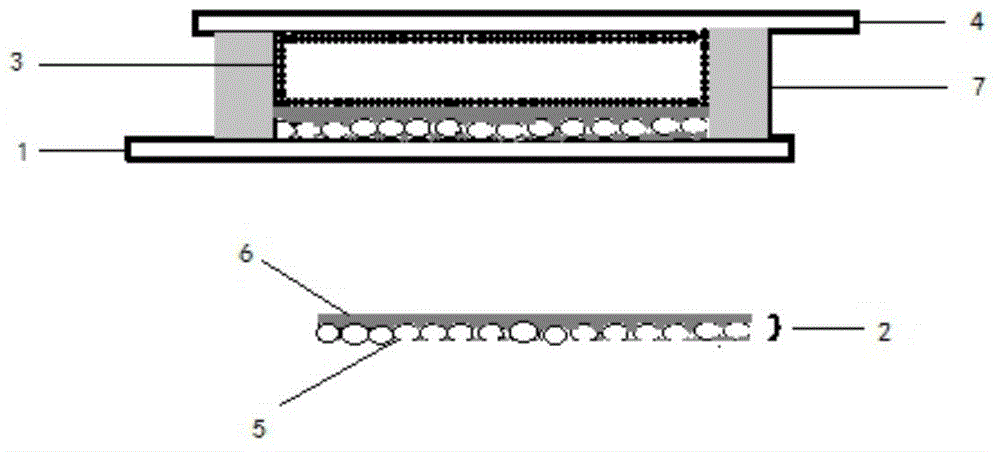
Poly(triphenylamine-benzothiophene/furan) dye and application thereof
A technology of benzothiophene and triphenylamine, which is used in the synthesis and application of functional dyes, can solve the problems of low photoelectric conversion efficiency, and achieve the effects of improving charge mobility, high current density, and improving migration efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
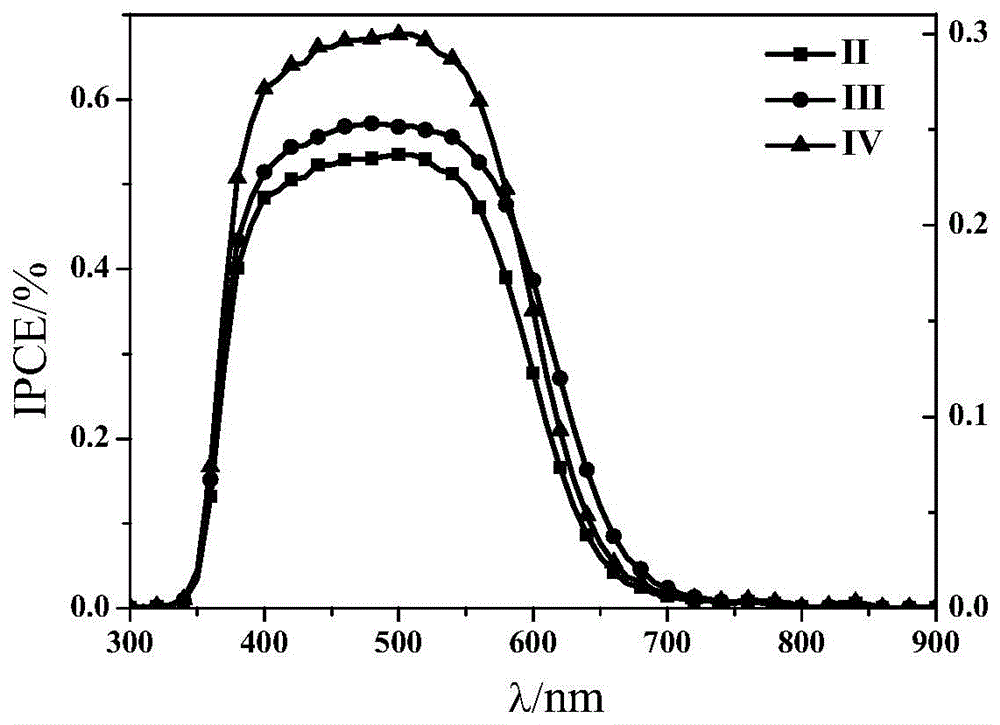
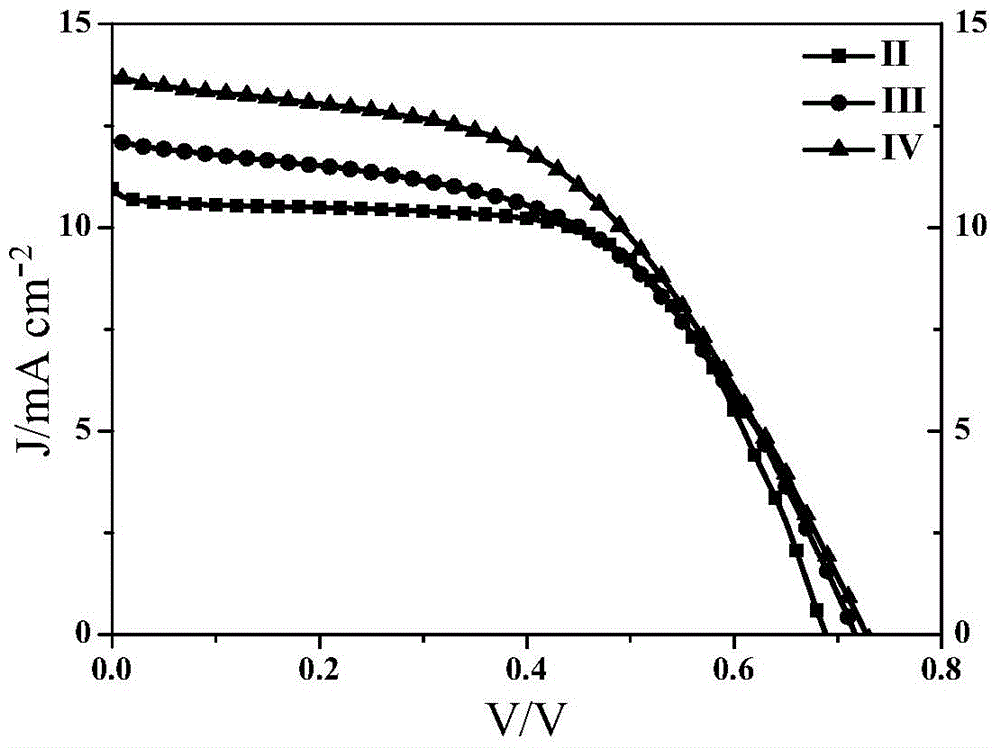
[0053] Synthesis of polymer dye sensitizer with formula II structural unit and its application in dye-sensitized solar cells.
[0054] The synthetic route is as follows:
[0055]
[0056] Synthesis of intermediate (2):
[0057] Add 12.96g 4-bromotriphenylamine, 12.4g 5-aldehyde-2-thiophene boronic acid, and 27.6g anhydrous potassium carbonate into the reaction flask, then add 100mL toluene, 100mL methanol, and add the catalyst ferrocene under the protection of nitrogen. Palladium chloride. Heat to 70°C and track the reaction to completion. The reaction was quenched by adding 150 mL of water and extracted with dichloromethane. The organic layers were combined, dried over anhydrous sodium sulfate, and filtered. The crude product was separated and purified by column chromatography (silica gel column, eluent: n-hexane / dichloromethane = 4 / 1) to obtain pure Intermediate 2 as a yellow solid with a yield of 60.2%.
[0058] NMR characterization data of intermediate (2):
[0059] 1 H NMR(CDC...
Embodiment 2
[0089] Synthesis of polymer dye sensitizers with structural units of formula III and application in dye-sensitized solar cells.
[0090] The synthetic route is as follows:
[0091]
[0092]
[0093] Synthesis of intermediate (2):
[0094] Add 12.96g 4-bromotriphenylamine, 12.4g 5-aldehyde-2-thiophene boronic acid, and 27.6g anhydrous potassium carbonate into the reaction flask, then add 100mL toluene, 100mL methanol, and add the catalyst ferrocene under the protection of nitrogen. Palladium chloride. Heat to 70°C and track the reaction to completion. The reaction was quenched by adding 150 mL of water and extracted with dichloromethane. The organic layers were combined, dried over anhydrous sodium sulfate, and filtered. The crude product was separated and purified by column chromatography (silica gel column, eluent: n-hexane / dichloromethane = 4 / 1) to obtain pure Intermediate 2 as a yellow solid with a yield of 60.2%.
[0095] NMR characterization data of intermediate (2):
[0096] ...
Embodiment 3
[0126] Synthesis of polymer dye sensitizer with structural unit of formula IV and its application in dye-sensitized solar cells.
[0127] The synthetic route is as follows:
[0128]
[0129] Synthesis of intermediate (2):
[0130] Add 12.96g 4-bromotriphenylamine, 12.4g 5-aldehyde-2-thiophene boronic acid, and 27.6g anhydrous potassium carbonate into the reaction flask, then add 100mL toluene, 100mL methanol, and add the catalyst ferrocene under the protection of nitrogen. Palladium chloride. Heat to 70°C and track the reaction to completion. The reaction was quenched by adding 150 mL of water and extracted with dichloromethane. The organic layers were combined, dried over anhydrous sodium sulfate, and filtered. The crude product was separated and purified by column chromatography (silica gel column, eluent: n-hexane / dichloromethane = 4 / 1) to obtain pure Intermediate 2 as a yellow solid with a yield of 60.2%.
[0131] NMR characterization data of intermediate (2):
[0132] 1 H NMR(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com