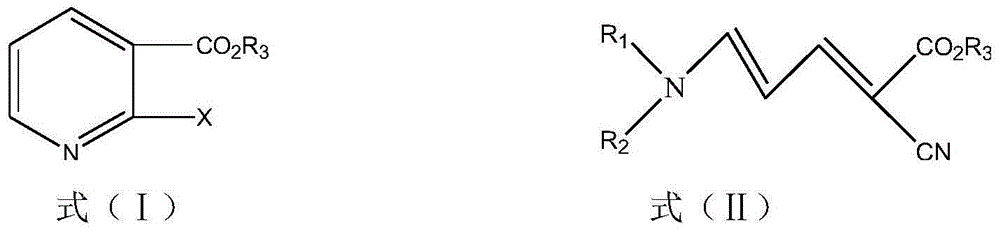Method for synthesizing 2-halogenated ester nicotinate and 2-halogenated ester nicotinate intermediate according to ultrasonic method
A technology of halogenated nicotinic acid ester and ultrasonic method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitrile, etc. It can solve the problems of unsuitable industrial production, unstable raw materials of malondialdehyde, and many wastes, etc. Problems, to achieve the effect of environmental protection, promote organic synthesis reaction, speed up the reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
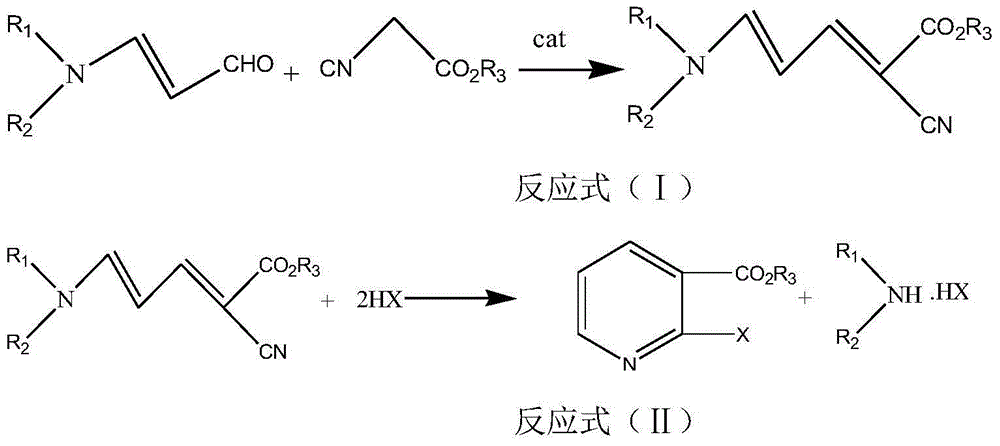
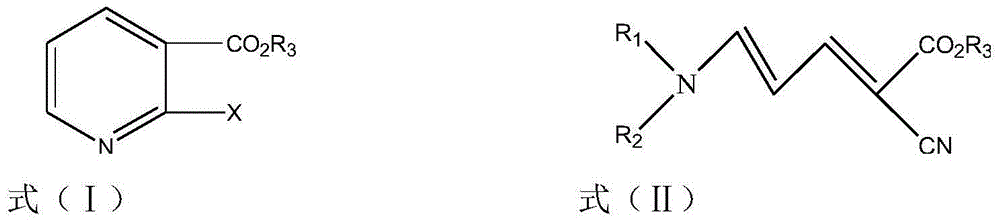
[0032] Example 1 5-(N,N-dimethyl)amino-2-cyano-2,4-pentadienoic acid ethyl ester
[0033] In a 500mL three-necked round-bottom flask, add 62mL (0.5mol) of 3-dimethylaminoacrolein, 10mL of triethylamine and 65mL (0.6mol) of ethyl cyanoacetate, and put the prepared device into a sonicator . Set the ultrasonic radiation conditions, react under the conditions of temperature 120°C, ultrasonic power 350W, and frequency 100KHz, high-performance liquid phase tracking and detection of the reaction process until the end of the reaction, add 10mL of deionized water to remove impurities, filter to obtain crude products, and then use 90.4 g of white powder was obtained by recrystallization from absolute ethanol, with a melting point of 134-135° C. and a product yield of 93.1%. The product has passed HRMS, 1 H NMR, 13 C NMR spectrum characterization, namely ethyl 5-(N,N-dimethyl)amino-2-cyano-2,4-pentadienoate. ESI-MS: m / z Calcd for C 10 h 14 N 2 o 2 217.0947[M+Na] + ,found 217.095...
Embodiment 2
[0037] Example 2 Butyl 5-(N,N-diethyl)amino-2-cyano-2,4-pentadienoate
[0038] In a 500 mL three-neck round bottom flask, add 61 mL (0.5 mol) of 3-diethylaminoacrolein, 10 mL of sodium ethoxide and 67 mL (0.6 mol) of butyl cyanoacetate, and put the prepared device into a sonicator. Set the ultrasonic radiation conditions, react under the conditions of temperature 100°C, ultrasonic power 300W, and frequency 80KHz, high-performance liquid phase tracking and detection of the reaction process until the end of the reaction, add 10mL of deionized water to remove impurities, and distill to obtain a light brown oil 113.5 g, yield 90.7%. The product was characterized by HRMS as butyl 5-(N,N-diethyl)amino-2-cyano-2,4-pentadienoate. ESI-MS: m / z Calcd for C 14 h 22 N 2 o 2 273.3265[M+Na] + ,found 273.3261[M+Na] + .
Embodiment 3
[0042] Example 3 Synthesis of ethyl 2-chloronicotinate
[0043] In a 500mL three-neck flask equipped with a thermometer, first add 62mL (0.5mol) of 3-dimethylaminoacrolein and 10mL of piperidine, then add 65mL (0.6mol) of ethyl cyanoacetate, and put the prepared device into the ultrasonic instrument. Set the ultrasonic radiation conditions, react under the conditions of temperature 100°C, ultrasonic power 250W and frequency 40KHz, TLC detection (petroleum ether: dichloromethane 1:2, sublimation iodine color development) 3-dimethylaminoacrolein The response is complete. Afterwards, HCl gas was introduced again, the conditions of ultrasonic radiation were as above, and the reaction was tracked by HPLC until the end of the reaction. Then after the reaction is over, add a mass fraction of 10% sodium hydroxide solution to adjust the pH=5-6, separate the layers, extract the aqueous layer with 20 mL of dichloromethane × 3 times, combine the organic layers, add anhydrous Na 2 SO 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com