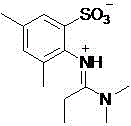
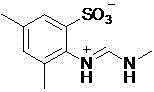
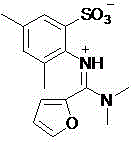
A sulfonic acid inner salt compound of amidine
A technology for sulfonic acid inner salts and compounds is applied in the design and synthesis of organic amidine salts, and achieves the effects of strong practical operability, wide sources and good atom economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment one: 3,5-dimethyl-2-aminobenzenesulfonyl chloride and N,N Synthesis of sulfonic acid inner salt compound of amidine by reaction of dimethylformamide
[0018] Add 219 mg 3,5-dimethyl-2-aminobenzenesulfonyl chloride (1 mmol) and 88 mg N,N - Dimethylformamide (1.2 mmol), stirred reaction at 25°C for 4 hours. After the reaction, add enough methanol and dichloromethane mixed solution to dissolve the reaction system into a homogeneous phase, add silica gel, remove the organic solvent under reduced pressure, and the residue is subjected to silica gel column chromatography (gradient elution, eluent is methanol / dichloromethane). Chloromethane system, the volume ratio is 1:5-1:30) to obtain 210 mg of white solid with a yield of 82%.
[0019] The structural formula of the obtained product and the main NMR test data are as follows, and it can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis;
[0020]
[0021]...
Embodiment 2
[0022] Embodiment two: 3,5-dimethyl-2-aminobenzenesulfonyl chloride and N,N Synthesis of sulfonic acid inner salt compound of amidine by reaction of dimethylformamide
[0023] Add 219 mg 3,5-dimethyl-2-aminobenzenesulfonyl chloride (1 mmol) and 110 mg N,N - Dimethylformamide (1.5 mmol), stirred reaction at 25°C for 2 hours. After the reaction, enough mixed solution of methanol and dichloromethane was added to dissolve the reaction system into a homogeneous phase, then silica gel was added, the organic solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography to obtain 233 mg of a white solid with a yield of 91%. . The NMR data of the product are the same as in Example 1.
Embodiment 3
[0024] Embodiment three: 3,5-dimethyl-2-aminobenzenesulfonyl chloride and N,N Synthesis of sulfonic acid inner salt compound of amidine by reaction of dimethylformamide
[0025] Add 219 mg 3,5-dimethyl-2-aminobenzenesulfonyl chloride (1 mmol) and 110 mg N,N - Dimethylformamide (1.5 mmol), stirred reaction at 25°C for 4 hours. After the reaction, enough mixed solution of methanol and dichloromethane was added to dissolve the reaction system into a homogeneous phase, silica gel was added, the organic solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography to obtain 254 mg of white solid with a yield of 99%. . The NMR data of the product are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com