Freeze-dried preparation containing fosaprepitant and preparation method of freeze-dried preparation
A freeze-dried preparation, fosaprepitant dimeglumine technology, applied in the field of medicine, can solve problems such as irritation of histamine release, secondary injury to patients, etc., and achieve the effects of improving compliance, low hemolysis rate, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
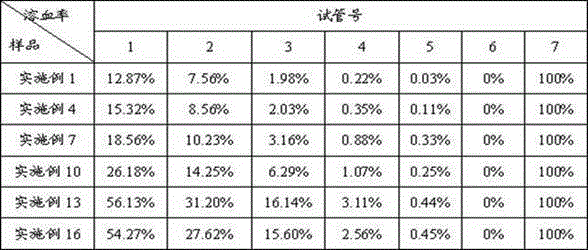
Examples
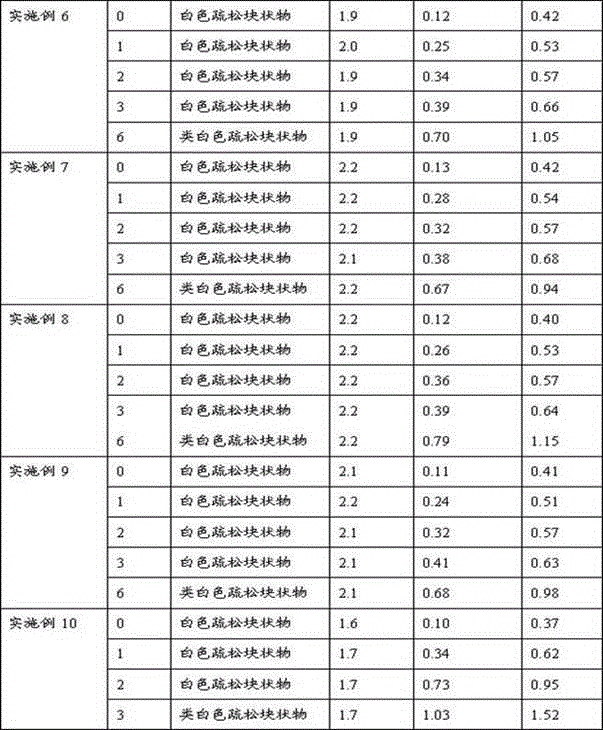
Embodiment 1
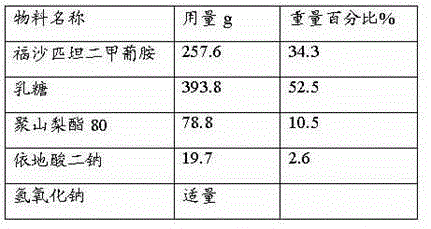
[0026] Embodiment 1: the preparation of fosaprepitant freeze-dried preparation
[0027] Prescription: 1000 sticks (calculated as fosaprepitant)
[0028]
[0029] Preparation:
[0030] Dissolve lactose and edetate disodium in 3500ml of water for injection, add 0.1% activated carbon (w / v), stir for 30 minutes, filter to remove carbon, then add polyethylene glycol lauryl lithium stearate, stir to dissolve, and then Add the main drug fosaprepitant dimeglumine and stir to dissolve. Use 2% sodium hydroxide solution to adjust the pH value of the liquid to 8.0-9.0, make up water for injection to 4000ml, add 0.05% activated carbon (w / v), stir for 30min, filter to remove carbon, sterilize and filter, subpackage, freeze-dry, Defusapitant freeze-dried preparation, the freeze-drying parameters are as follows: the vial is heat-treated at about -40~-30°C for about 2~4 hours, then the plate layer of the freeze dryer is heated to about -20~-10°C, and the low-pressure freeze-drying The c...
Embodiment 2~3
[0031] Embodiment 2~3: the investigation of solubilizer consumption
[0032] With reference to the prescription and preparation process thereof of Example 1, only the dosage of the solubilizing agent is changed to 40g and 80g respectively.
Embodiment 4~15
[0033] Embodiment 4~15: the investigation of solubilizing agent kind
[0034] Referring to the prescription and preparation process of Examples 1, 2 and 3, only the solubilizing agent was changed to hydroxypropyl-β-cyclodextrin, polyethylene glycol 400, poloxamer 188, and lecithin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com