Medicine composition and application of medicine composition to preparing medicines for preventing and treating atherosclerosis and dyslipidemia
A technology of dyslipidemia and composition, applied in the field of medicine, can solve the problems of no obvious advantage in long-term curative effect, unsatisfactory curative effect, side effects, etc., achieve improvement of aortic lesion, good synergistic anti-atherosclerosis, and increase activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
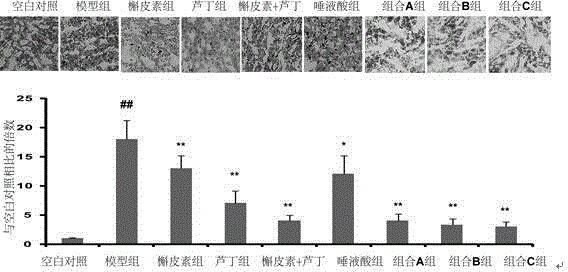
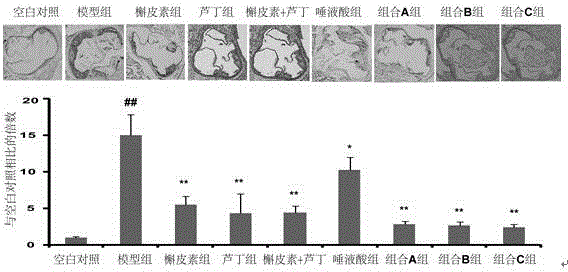
[0020] Therapeutic effects of quercetin and N-acetylneuraminic acid on atherosclerosis model mice
[0021] SPF grade apolipoprotein E knockout (apoE- / -) mice, male, weighing 20±2 g. Under the experimental conditions, the normal feed was given adaptive feeding for 1 week, and the experimental animals were randomly divided into 5 groups: 12 animals in the blank group, 15 animals in the model group, 15 animals in the quercetin group, 15 animals in the N-acetylneuraminic acid group and the combination group. A group of 15. Except for the blank group, all rats were given high-fat feed (formula: cholesterol 1.25%, bile salt 0.5%, lard 10%, egg yolk powder 10%, basal feed 78.25%); the blank group was given normal feed. After feeding for 1 month, each group was given the corresponding drug by gavage at 0.2 mL / kg under the condition of unchanged feed, and the blank group and model group were given the same volume of normal saline, once a day, for 2 consecutive months. The test drugs ...
Embodiment 2
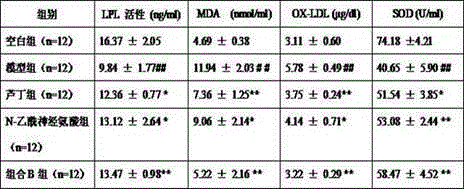
[0030] Treatment of rutin and N-acetylneuraminic acid on atherosclerosis model mice
[0031] SPF grade apolipoprotein E knockout (apoE- / -) mice, male, weighing 20±2 g. Under the experimental conditions, the normal feed was given adaptive feeding for 1 week, and the experimental animals were randomly divided into 5 groups: 12 animals in the blank group, 12 animals in the model group, 12 animals in the rutin group, 12 animals in the N-acetylneuraminic acid group and combination B Group of 12. Except for the blank group, all rats were given high-fat feed (formula: cholesterol 1.25%, bile salt 0.5%, lard 10%, egg yolk powder 10%, basal feed 78.25%); the blank group was given normal feed. After feeding for 1 month, each group was given the corresponding drug by gavage at 0.2 mL / kg under the condition of unchanged feed, and the blank group and model group were given the same volume of normal saline, once a day, for 2 consecutive months. The test drugs and doses of each group are a...
Embodiment 3
[0041] Treatment of atherosclerosis model mice with the combination of quercetin and rutin combined with N-acetylneuraminic acid
[0042] SPF grade apolipoprotein E knockout (apoE- / -) mice, male, weighing 20±2 g. Under the experimental conditions, the animals were given adaptive feeding with ordinary feed for 1 week, and the experimental animals were randomly divided into 5 groups: 12 animals in the blank group, 12 animals in the model group, 12 animals in the mixed quercetin rutin group, and 12 animals in the N-acetylneuraminic acid group. Only and combination C group 12. Except for the blank group, all rats were given high-fat feed (formula: cholesterol 1.25%, bile salt 0.5%, lard 10%, egg yolk powder 10%, basal feed 78.25%); the blank group was given normal feed. After feeding for 1 month, each group was given the corresponding drug by gavage at 0.2 mL / kg under the condition of unchanged feed, and the blank group and model group were given the same volume of normal saline,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com