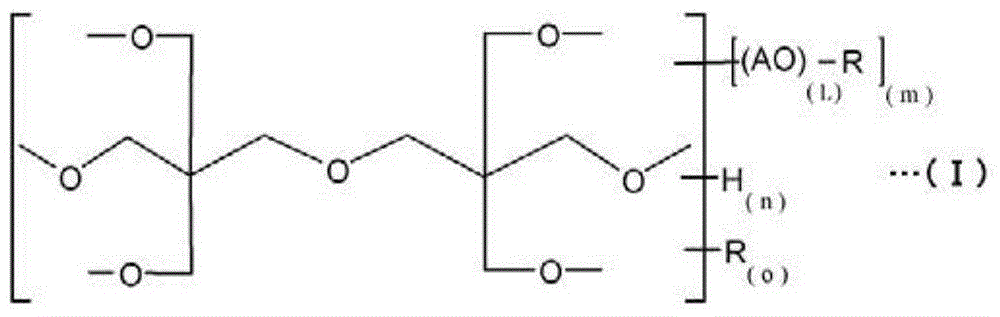
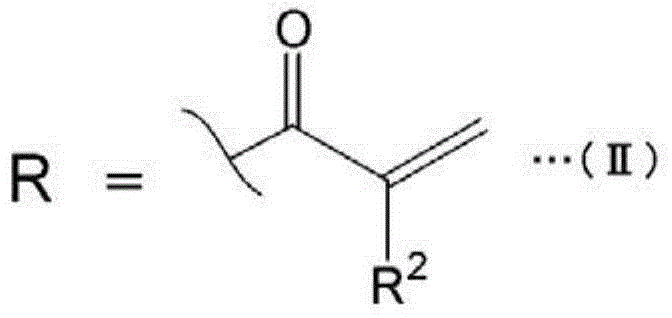
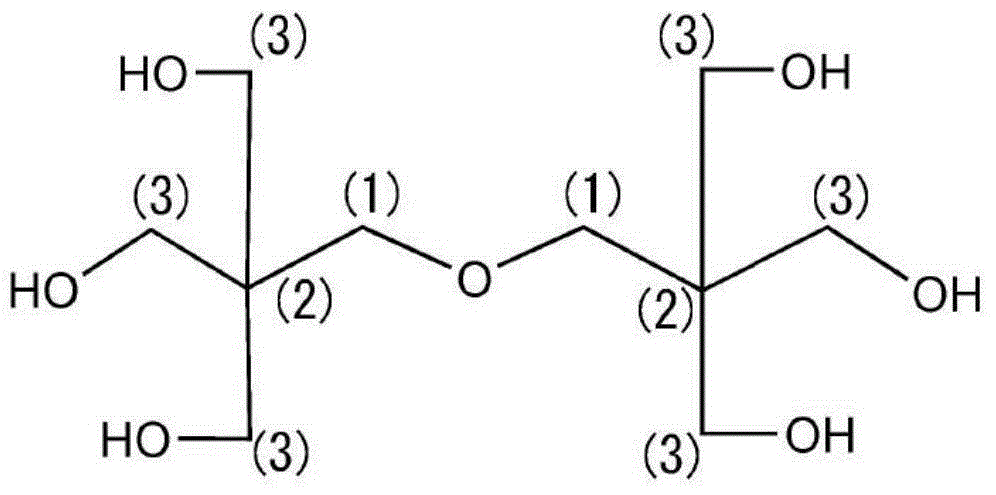
Ink composition for inkjet printing
An inkjet printing and composition technology, applied in printing, ink, application, etc., can solve the problems of insufficient hardness and friction resistance of hardened products, and reduced crosslinking density, and achieve low shrinkage, maintain film strength, and produce excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] [Example 1] (composition containing dipentaerythritol 3EO adduct acrylate)
[0082]
[0083] Add 254 g (1.0 mol) of dipentaerythritol (manufactured by Guangei Chemical Industry Co., Ltd., OH value 1324), 127 g of toluene, and 0.3 g of KOH to a 1 L autoclave equipped with a stirring device, and heat up to 90° C. Stir to make a slurry. Next, it heated to 130 degreeC, and 176 g (4 mol) of ethylene oxides were introduced into an autoclave slowly, and it was made to react. With the introduction of ethylene oxide, the temperature inside the autoclave rose. Cooling was performed as needed to keep the reaction temperature at 140°C or lower. After the reaction, excess ethylene oxide and polymers of by-produced ethylene glycol were removed by reducing the pressure at 140° C. to a mercury column of 10 mmHg or less. Thereafter, neutralization was performed with acetic acid, and the pH was adjusted to 6-7. The OH value of the obtained dipentaerythritol 3EO adduct was 897.
[...
Embodiment 2
[0096] [Example 2] (composition containing dipentaerythritol 4EO adduct acrylate)
[0097]
[0098] In an autoclave with a capacity of 1 L equipped with a stirring device, 254 g (1.0 mol) of dipentaerythritol (manufactured by Guangrong Chemical Industry Co., Ltd., OH value 1324), 127 g of toluene, and 0.3 g of KOH were added, and the temperature was raised to 90° C. and stirred to prepare Into a slurry liquid. Next, it heated to 130 degreeC, and 220 g (5 mol) of ethylene oxides were introduced into an autoclave slowly, and it was made to react. With the introduction of ethylene oxide, the temperature inside the autoclave rose. Cooling was performed as needed to keep the reaction temperature at 140°C or lower. After the reaction, excess ethylene oxide and polymers of by-produced ethylene glycol were removed by reducing the pressure at 140° C. to a mercury column of 10 mmHg or less. Thereafter, neutralization was performed with acetic acid, and the pH was adjusted to 6-7. ...
Embodiment 3
[0109] [Example 3] (composition containing dipentaerythritol 5EO adduct acrylate)
[0110]
[0111] In an autoclave with a capacity of 1 L equipped with a stirring device, 254 g (1.0 mol) of dipentaerythritol (manufactured by Guangrong Chemical Industry Co., Ltd., OH value 1324), 36 g of distilled water, and 0.3 g of KOH were added, and the temperature was raised to 90° C. and stirred to prepare Into a slurry liquid. Next, it heated to 130 degreeC, and 264 g (6 mol) of ethylene oxides were introduced into an autoclave slowly, and it was made to react. With the introduction of ethylene oxide, the temperature inside the autoclave rose. Cooling was performed as needed to keep the reaction temperature at 140°C or lower. After the reaction, excess ethylene oxide and polymers of by-produced ethylene glycol were removed by reducing the pressure at 140° C. to a mercury column of 10 mmHg or less. Thereafter, neutralization was performed with acetic acid, and the pH was adjusted to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com