Self-supporting transitional metal sulfide catalyst and preparation methods and applications thereof
A technology of transition metals and transition metal elements, applied in the field of self-supporting transition metal sulfide catalysts and their preparation, can solve the problems of limited pH value and low catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention also provides a preparation method of a self-supporting transition metal sulfide catalyst, the method comprising:
[0024] Step 1: mixing metal salts of transition metal elements and sulfur sources, or adding surfactants, catalyst carriers, and alkaline solutions to obtain mixed solutions;
[0025] Step 2: adding the transition metal conductive substrate into the mixed solution obtained in Step 1 for reaction to obtain a self-supporting transition metal sulfide catalyst.
[0026] According to the present invention, the metal salt of the transition metal element and the sulfur source are mixed, or a surfactant is added, or a catalyst carrier is added, or an alkaline solution is added, preferably dissolved in distilled water under the condition of magnetic stirring, to obtain a mixed solution;
[0027] The sulfur source is preferably thiourea, thioacetamide, cysteine or sodium sulfide. The metal salt of the transition metal element is preferably o...
Embodiment 1
[0039] Weigh 1 mmol of zinc nitrate, 2 mmol of cobalt nitrate, 2 mmol of ammonium fluoride, and 35 milliliters of 3 mmol of thiourea in aqueous solution and mix to obtain a mixed solution; the titanium mesh is cleaned with dilute hydrochloric acid, ethanol, and deionized, and then Adding to the above mixed solution, reacting at 300° C. for 0.5 hour to obtain a zinc cobalt sulfur nanowire array.
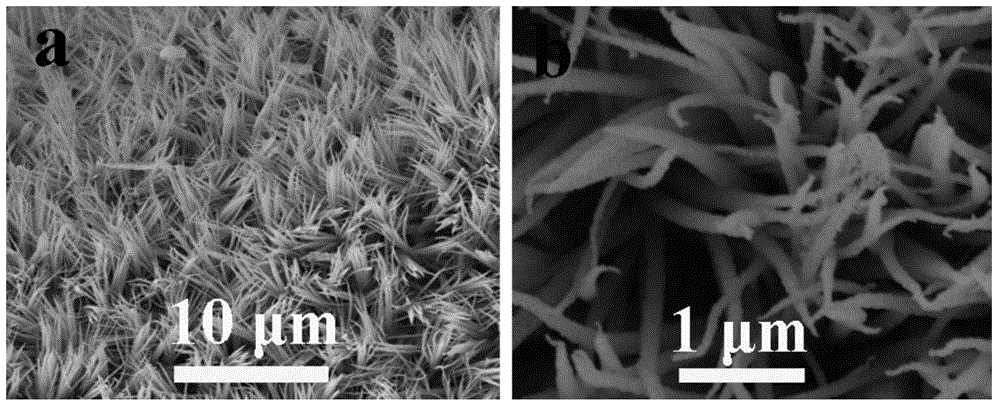
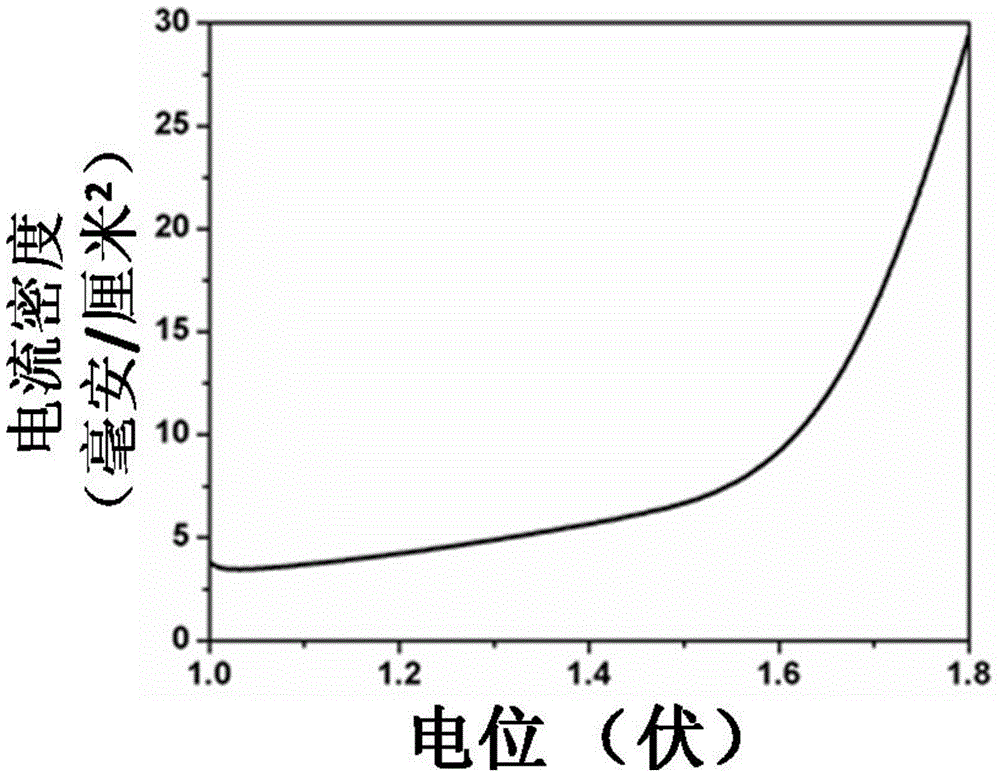
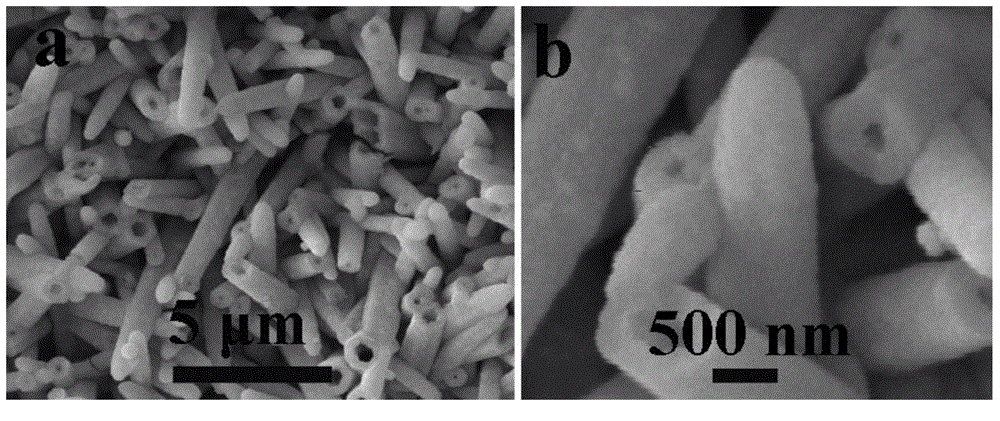
[0040] figure 1 It is the scanning electron micrograph picture of the zinc-cobalt-sulfur nanowire array prepared in Example 1, wherein picture a is a scanning electron micrograph under a 10 μm scale, and figure b is a scanning electron micrograph under a 10 μm scale, which shows that the titanium mesh is The zinc-cobalt-sulfur nanowire array is completely covered, with a diameter of 80-200 nanometers and a length of 2-6 microns. figure 2 The (1 mole of potassium hydroxide, pH=14) polarization curve of the zinc-cobalt-sulfur nanowire array prepared in Example 1 under alkaline conditi...
Embodiment 2
[0042] Weigh 5mmol of nickel nitrate, 10mmol of cobalt nitrate, 4mmol of ammonium fluoride, and 35ml of 5mmol of cysteine in water and mix to obtain a mixed solution; wash the titanium sheet with dilute hydrochloric acid, ethanol, and deionized , and then added to the above mixed solution, and reacted at 180° C. for 5 hours to obtain a nickel-cobalt-sulfur nanowire array.
[0043] The nickel-cobalt-sulfur nanowire array obtained in Example 2 was tested for hydrogen evolution performance, when j=10mA cm -2 The potential of the nickel-cobalt-sulfur nanowire catalyst electrode relative to the standard hydrogen electrode is 1.68 volts.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com