Anti-cancer drug erlotinib hydrochloride tablet and preparation method thereof
A technology of erlotinib hydrochloride tablets and anticancer drugs, which is applied in the field of pharmaceutical preparations, can solve the problems of erlotinib hydrochloride solubilization, slow disintegration of tablets, and drug stability, and achieve rapid dissolution, Less toxic and side effects, avoiding gastrointestinal side effects
- Summary
- Abstract
- Description
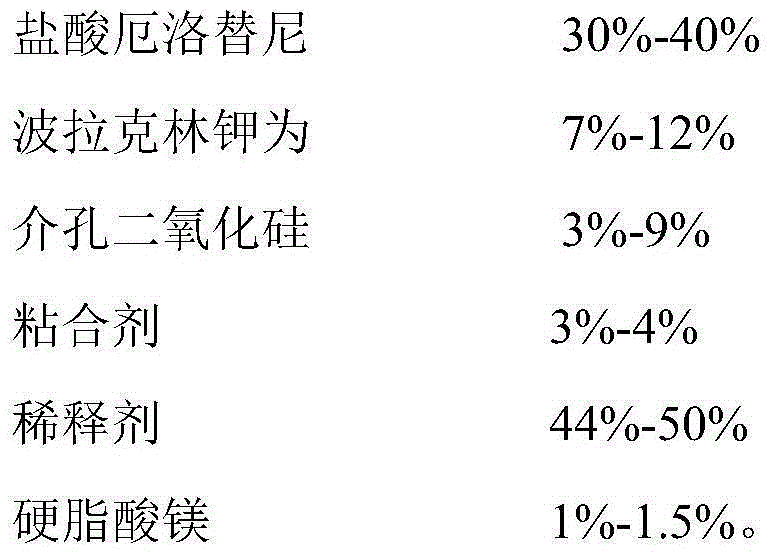
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033]
[0034] Preparation:
[0035] (1) Add hydroxypropyl cellulose to pH2 hydrochloric acid solution, stir until completely dissolved, add erlotinib hydrochloride and stir evenly, grind through a colloid mill, and control the particle size to D 90 ≤30 microns, spare;
[0036] (2) Weigh Polacrilin Potassium IRP-88, mesoporous silica, lactose, and starch into the fluidized bed, and mix evenly; spray the mixed solution prepared in step (1) to granulate, and the air inlet temperature is 60°C. The speed of the injection pump is 10rpm, the atomization pressure is 0.5Mpa, after the liquid injection is completed, continue to dry for 30min, and pass the dry particles through a 20-mesh sieve for granulation;
[0037] (3) Mix the dry granules prepared in step (2) with magnesium stearate evenly, punch the tablets with a shallow arc with a diameter of 9 mm, and control the average hardness to 8-10 kg / m 2 , that is.
Embodiment 2
[0039]
[0040] Preparation:
[0041](1) Add hydroxypropyl cellulose to pH2 hydrochloric acid solution, stir until completely dissolved, add erlotinib hydrochloride and stir evenly, grind through a colloid mill, and control the particle size to D 90 ≤30 microns, spare;
[0042] (2) Weigh Polacrilin Potassium IRP-88, mesoporous silica and microcrystalline cellulose into the fluidized bed, mix evenly; spray the mixed liquid prepared in step (1) to granulate, and the air inlet temperature is 60°C , the speed of the injection pump is 10rpm, the atomization pressure is 0.5Mpa, after the liquid injection is completed, continue to dry for 30min, and pass the dry particles through a 20-mesh sieve for granulation;
[0043] (3) Mix the dry granules prepared in step (2) with sodium stearate fumarate evenly, punch the tablets with a shallow arc with a diameter of 10 mm, and control the average hardness to 8-10 kg / m 2 , that is.
Embodiment 3
[0045]
[0046]
[0047] Preparation:
[0048] (1) Add hydroxypropyl cellulose to pH2 hydrochloric acid solution, stir until completely dissolved, add erlotinib hydrochloride and stir evenly, grind through a colloid mill, and control the particle size to D 90 ≤30 microns, spare;
[0049] (2) Weigh Polacrilin Potassium IRP-88, mesoporous silica and mannitol into the fluidized bed and mix evenly; spray the mixed solution prepared in step (1) to granulate, the inlet air temperature is 60°C, and The rotation speed of the sample pump is 10rpm, the atomization pressure is 0.5Mpa, after the liquid feeding is completed, continue to dry for 30min, and the dry particles pass through a 20-mesh sieve for granulation;
[0050] (3) Mix the dry granules prepared in step (2) with magnesium stearate evenly, punch the tablets with a shallow arc with a diameter of 10mm, and control the average hardness to 8-10kg / m 2 , that is.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com