Recombinant human interferon alpha 2b gel and preparation method thereof
A technology of recombinant human interferon and gel, which is applied in the directions of drug combinations, pharmaceutical formulations, medical preparations of inactive ingredients, etc. Obvious effect, good adhesion and uniformity, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
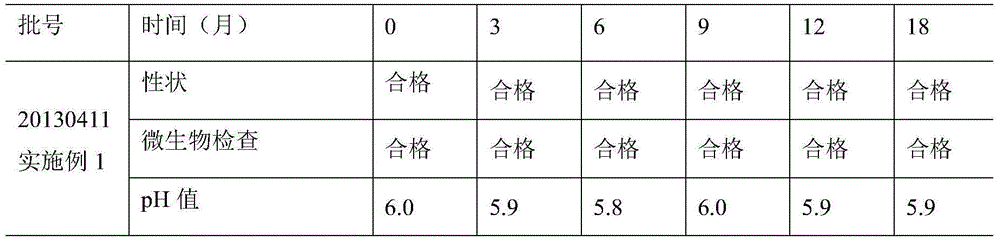
[0020] Embodiment 1: Preparation of recombinant human interferon α2b gel of the present invention
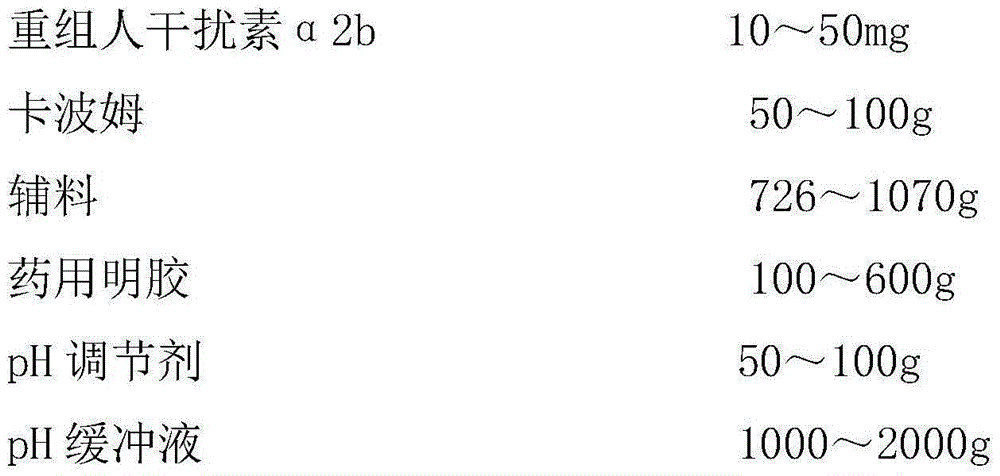
[0021] a) Weigh 50g carbomer, add 100 times the weight of water for injection to swell overnight;
[0022] b) Weigh 200g of propylene glycol, 20g of Tween 80, 1g of disodium EDTA, 500g of glycerin, and 5g of ethylparaben, mix them, add 2 times the weight of water for injection and heat and stir in a water bath at 75°C until ethylparaben Dissolve completely, add the solution to the swollen carbomer matrix solution, mix well, add triethanolamine to adjust the pH to 5.0, sterilize with moist heat for 1 hour, cool and balance to 10°C, and obtain the carbomer matrix for use;
[0023] c) Weigh 600g of medicinal gelatin, dissolve it with 2L phosphate buffer (pH5.0, concentration 0.1M, temperature 20°C), and then place it in a sterilizing pot at a pressure of 0.1MPa and a temperature of 121°C Sterilize for 30min, cool naturally to 10°C, add 50.0mg recombinant human interferon α2b (1.0×...
Embodiment 2
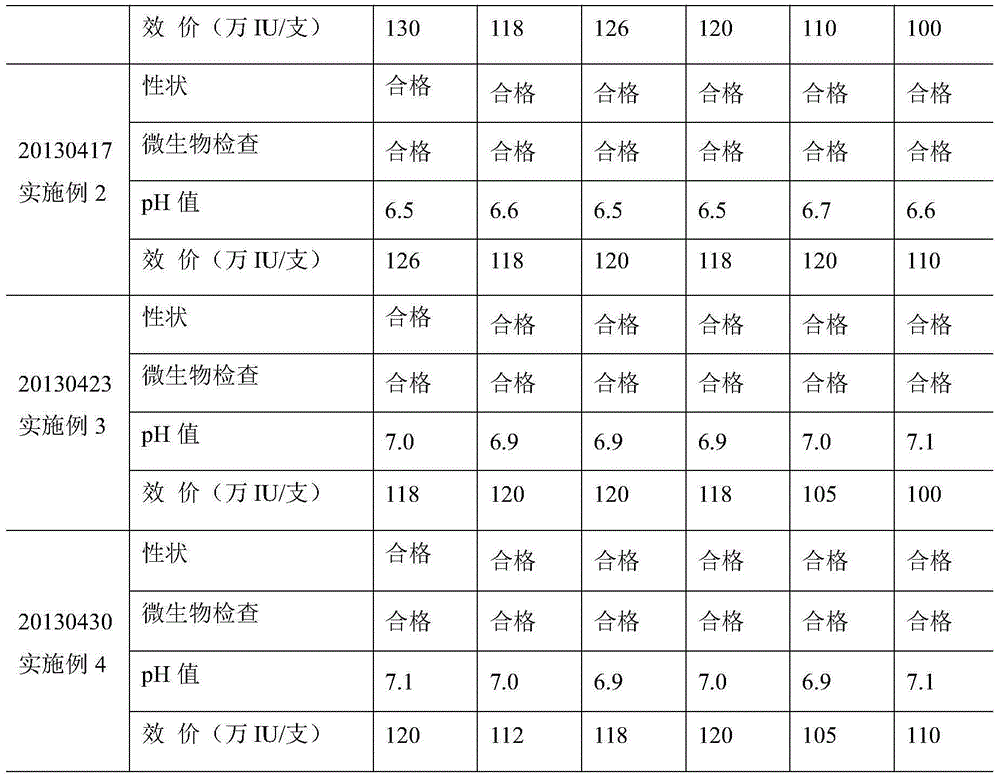
[0025] Embodiment 2: the preparation of recombinant human interferon α2b gel of the present invention
[0026] a) Weigh 80g carbomer, add 50 times the weight of water for injection to swell overnight;
[0027]b) Weigh 500g of propylene glycol, 50g of Tween 80, 5g of disodium EDTA, 500g of glycerin, and 7g of ethylparaben, mix them, add 1 times the weight of water for injection and heat and stir in a water bath at 80°C until ethylparaben Dissolve completely, add the solution to the swollen carbomer matrix solution, mix well, add triethanolamine to adjust the pH to 6.0, sterilize with moist heat for 2 hours, cool and balance to 20°C, and obtain the carbomer matrix for use;
[0028] c) Weigh 200g of medicinal gelatin, dissolve it with 1L phosphate buffer (pH6.5, concentration 0.2M, temperature 25°C), and then place it in a sterilizing pot under the conditions of a pressure of 0.05MPa and a temperature of 130°C Sterilize for 30min, cool naturally to 20°C, add 20.0mg recombinant h...
Embodiment 3
[0030] Embodiment 3: the preparation of recombinant human interferon α2b gel of the present invention
[0031] a) Weigh 100g carbomer, add 60 times the weight of water for injection to swell overnight;
[0032] b) Weigh 400g of propylene glycol, 100g of Tween 80, 10g of disodium EDTA, 500g of glycerin, and 10g of ethylparaben, mix them, add 1 times the weight of water for injection, and heat and stir in a water bath at 70°C until ethylparaben Dissolve completely, add the solution to the swollen carbomer matrix solution, mix well, add triethanolamine to adjust the pH to 7.0, sterilize with moist heat for 0.5 hours, cool and balance to 5°C, and obtain the carbomer matrix for use;
[0033] c) Weigh 100g of medicinal gelatin, dissolve it in 1L of phosphate buffer (pH7.5, concentration 0.4M, temperature 20°C), and then place it in a sterilizer under the conditions of a pressure of 0.05MPa and a temperature of 130°C Sterilize for 30min, cool naturally to 5°C, add 10.0mg recombinant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com