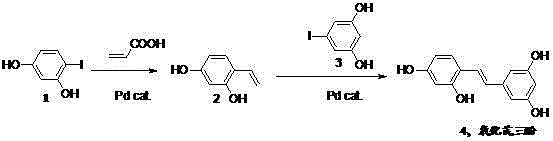
Synthetic method of oxyresveratrol
A technology of oxystilbene triphenol and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of long synthesis route, high risk of operation, low total yield, etc. Short, less reaction steps, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the synthesis of oxystilbene triphenol
[0017] Add 0.014g lithium chloride, 0.200g tetrakis (triphenylphosphine) palladium, 0.324g potassium hydroxide, 0.880g 4-iodine resorcinol, 0.300g acrylic acid in sequence in a 50mL round bottom flask, then add 3mL distilled water, Heat and stir in an oil bath at 85°C for 3h. The reaction system was cooled to room temperature. Then add 0.005g lithium chloride, 0.145g tetrakis (triphenylphosphine) palladium, 0.900g 5-iodine resorcinol, 0.82mL piperidine, 12mL N,N -Dimethylacetamide, heating and reacting in an oil bath at 140°C for 14h. After the reaction was completed, the system was cooled to room temperature and filtered. Dilute hydrochloric acid was added to the filtrate to make it acidic (pH=5). Pour into a separatory funnel and extract with ethyl acetate (20mL*3). Combine the organic phases, dry the organic layer with anhydrous sodium sulfate, filter, and spin off the solvent. The crude product was purifie...
Embodiment 2
[0018] Embodiment 2: the synthesis of oxystilbene triphenol
[0019] Add 0.140g of lithium chloride, 2.000g of tetrakis(triphenylphosphine) palladium, 3.240g of potassium hydroxide, 8.800g of 4-iodoresorcinol, 3.000g of acrylic acid in sequence in a 500mL round bottom flask, and then add 30mL of distilled water. Heat and stir in an oil bath at 85°C for 3.5 hours. The reaction system was cooled to room temperature. Then add 0.050g lithium chloride, 1.450g tetrakis(triphenylphosphine) palladium, 9.000g 5-iodoresorcinol, 8.20mL piperidine, 120mL N,N -Dimethylacetamide, heating and reacting in an oil bath at 140° C. for 15 hours. After the reaction was completed, the system was cooled to room temperature and filtered. Dilute hydrochloric acid was added to the filtrate to make it acidic (pH=5). Pour into a separatory funnel and extract with ethyl acetate (200mL*3). Combine the organic phases, dry the organic layer with anhydrous sodium sulfate, filter, and spin off the solvent...
Embodiment 3
[0020] Embodiment 3: the synthesis of oxystilbene triphenol
[0021] Under the protection of nitrogen, add 0.014g lithium chloride, 0.200g tetrakis (triphenylphosphine) palladium, 0.324g potassium hydroxide, 0.880g 4-iodine resorcinol, 0.300g acrylic acid to a 50mL round bottom flask successively, and then add 3mL of distilled water was heated and stirred in an oil bath at 85°C for 3h. The reaction system was cooled to room temperature. Then add 0.005g lithium chloride, 0.145g tetrakis (triphenylphosphine) palladium, 0.900g 5-iodine resorcinol, 0.82mL piperidine, 12mL N,N -Dimethylacetamide, heating and reacting in an oil bath at 140°C for 14h. After the reaction was completed, the system was cooled to room temperature and filtered. Dilute hydrochloric acid was added to make the filtrate acidic (pH=5). Pour into a separatory funnel and extract with ethyl acetate (25mL*3). Combine the organic phases, dry the organic layer with anhydrous sodium sulfate, filter, and spin off...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com