Continuous synthesis method of cyclohexane polyacid ester
A polybasic acid ester and cyclohexane technology, which is applied in the field of synthesizing cyclohexane polybasic ester, can solve the problems of large energy consumption and cumbersome steps, and achieve the effects of energy saving, production cost reduction, time and energy saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
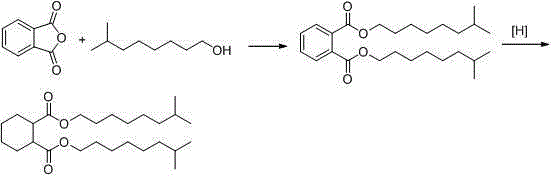
[0032] Add phthalic anhydride and isononyl alcohol with a molar ratio of 1:2.5 into the stirred tank reactor, and the reactor uses an alcohol-water reflux layering device, and then adds an acid catalyst and heats to 220°C for esterification reaction, as shown in the formula Shown in I, react to the acid value of diisononyl phthalate crude product below 0.2mgKOH / g, gained diisononyl phthalate crude product contains excess isononyl alcohol (containing about 85% phthalate diisononyl formate and approximately 15% isononyl alcohol). Take 3.9g of X catalyst and fill it in a stainless steel continuous trickle bed reactor with a diameter of 3 / 8 inches as a fixed bed (filling 6mL), and then directly feed the crude product of the esterification reaction without purification To the trickle bed reactor for hydrogenation reaction, as shown in formula I, the hydrogen pressure is 50bar, the temperature is 70°C, the hydrogen flow rate is 100mL / min, the esterification reaction crude product fl...
Embodiment 2
[0038]Add phthalic anhydride and isononyl alcohol with a molar ratio of 1:2.5 into the stirred tank reactor. The reactor uses an alcohol-water reflux layering device, and then adds an acid catalyst and heats it to 220°C for esterification reaction. The acid value of the crude diisononyl phthalate is lower than 0.2 mgKOH / g, and the obtained crude diisononyl phthalate contains excess isononyl alcohol. Take 3.9g of X catalyst and fill it in a stainless steel continuous trickle bed reactor with a diameter of 3 / 8 inches as a fixed bed (filling 6mL), and then directly feed the crude product of the esterification reaction without purification To the trickle bed reactor for hydrogenation reaction, the hydrogen pressure is 50bar, the temperature is 100°C, the hydrogen flow rate is 100mL / min, the esterification reaction crude product flow rate is 4.5mL / h, the hydrogen and diisononyl phthalate The molar ratio is about 28:1. After the reaction, add alkali to neutralize and remove acid, d...
Embodiment 3
[0041] Add phthalic anhydride and isononyl alcohol with a molar ratio of 1:2.5 into the stirred tank reactor. The reactor uses an alcohol-water reflux layering device, and then adds an acid catalyst and heats it to 220°C for esterification reaction. The acid value of the crude diisononyl phthalate is lower than 0.2 mgKOH / g, and the obtained crude diisononyl phthalate contains excess isononyl alcohol. Take 3.9g of X catalyst and fill it in a stainless steel continuous trickle bed reactor with a diameter of 3 / 8 inches as a fixed bed (filling 6mL), and then directly feed the crude product of the esterification reaction without purification To the trickle bed reactor for hydrogenation reaction, the hydrogen pressure is 50bar, the temperature is 120°C, the hydrogen flow rate is 100mL / min, the crude product flow rate of the esterification reaction is 4.5mL / h, the hydrogen and diisononyl phthalate The molar ratio is about 28:1. After the reaction, add alkali to neutralize and remove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com