A kind of norisopoldin chitosan microsphere and preparation method thereof
A technology of meisobolidine chitosan acetate and norisobolidine, which is applied in the direction of bone diseases, non-central analgesics, antipyretics, etc., can solve the problem of low bioavailability, and achieve the goal of reducing drug consumption. The effect of dosage, good application prospect and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
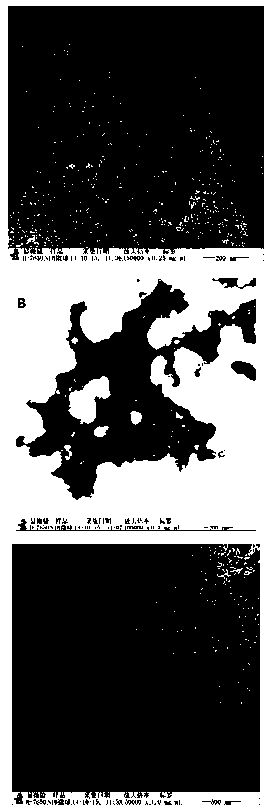
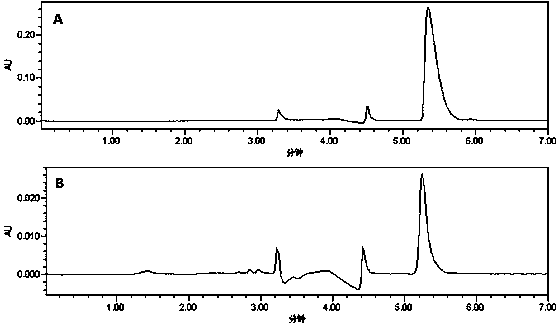
Image
Examples
Embodiment 1
[0029] Experimental raw materials:
[0030] Norisoboldine (provided by Shanghai Traditional Chinese Medicine Standardization Research Center, purity > 98%); chitosan (Shanghai Boao Biotechnology Co., Ltd., batch number 130204, deacetylation degree ≥ 90.0%, viscosity < 100cps); acetonitrile, Formic acid (Merck, chromatographically pure); acetic acid (Sinopharm Chemical Reagent Co., Ltd., analytically pure); liquid paraffin (Sinopharm Chemical Reagent Co., Ltd., analytically pure), ethyl acetate, petroleum ether (manufactured by Tianjin Hengxing Chemical Reagent Co., Ltd. Co., Ltd., analytically pure); Span-80, Tween-20, Tween-80 (Sinopharm Chemical Reagent Co., Ltd., chemically pure); glutaraldehyde (Aladdin Industrial Company, with a mass fraction of 50%, chemically pure ); water is ultrapure water.
[0031] Main instruments:
[0032] Waters Alliance high performance liquid chromatograph; Waters 2998 PAD detector; H7650 transmission electron microscope (TEM) from HITACH comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com