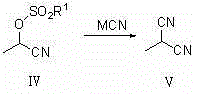
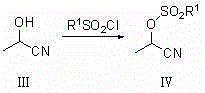
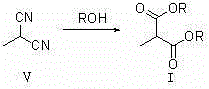Synthetic method of 2-diester methylmalonate compounds
A methylmalonate diester and synthetic method technology, applied in the preparation of organic compounds, chemical instruments and methods, and carboxylate preparation, can solve the problems of increasing the difficulty of separation and purification, the difficulty of product separation, and the high cost of raw materials. Achieve the effect of unique method, simple post-processing and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of A, 2-hydroxypropionitrile:
[0035]
[0036] Add acetaldehyde (44.05g, 1mol) to the four-neck flask, cool down to 0-10°C, then add hydrocyanic acid (32.43g, 1.2mol) dropwise, stir the reaction, add triethylamine dropwise to control the pH at 2~ 3. Insulation reaction for 1-2h. The reaction system makes free HCN escape from the reaction solution under vacuum conditions, and the tail gas of 30% NaOH solution is absorbed. After removing HCN, 70.75 g of 2-hydroxypropionitrile was obtained, with a yield of 99.54%. 1 HNMR (300MHz, CDCl 3 ): 1.56~1.60 (m,3H,CH 3 ), 4.43~4.49 (m, H, CH).
[0037] B, the synthesis of 2-cyanoethyl methanesulfonate:
[0038]
[0039] Add 2-hydroxypropionitrile (71.08g, 1mol), triethylamine (101.19g, 1mol) and 100mL of dichloromethane into a four-neck flask, cool down to 0-10°C, and slowly add methanesulfonyl chloride (114.55g , 1mol), and reacted at 20-25°C for 24h after dropping. Add 30 mL of saturated sodium carbonate s...
Embodiment 2
[0047] Synthesis of A, 2-hydroxypropionitrile:
[0048] Add acetaldehyde (44.05g, 1mol) to the four-neck flask, cool down to 0-10°C, then add hydrocyanic acid (54.06, 2.0mol) dropwise, stir the reaction, add dropwise 50% sodium hydroxide solution to control the pH at 8-9, heat preservation reaction for 3-4h. The reaction system makes free HCN escape from the reaction solution under vacuum conditions, and the tail gas of 30% NaOH solution is absorbed. After removing HCN, 70.60 g of 2-hydroxypropionitrile was obtained, with a yield of 99.32%.
[0049] B, the synthesis of 2-cyanoethyl trifluoromethanesulfonate:
[0050]
[0051] Add 2-hydroxypropionitrile (71.08g, 1mol), potassium carbonate (276.42g, 2mol) and 150mL dichloroethane into the four-neck flask, cool down to 0-10°C, and slowly add trifluoromethanesulfonyl chloride ( 219.08g, 1.3mol), reacted at 20~25℃ for 20h after dropping. After filtering to remove salt, add 80 mL of saturated sodium carbonate solution to rins...
Embodiment 3
[0058] Synthesis of A, 2-hydroxypropionitrile:
[0059] Add acetaldehyde (44.05g, 1mol) to the four-neck flask, cool down to 0-10°C, then add hydrocyanic acid (27.03, 1.0mol) dropwise, stir the reaction, add pyridine dropwise to control the pH at 4-5, keep warm Reaction 2-3h. The reaction system makes free HCN escape from the reaction solution under vacuum conditions, and the tail gas of 30% NaOH solution is absorbed. After removing HCN, 70.05 g of 2-hydroxypropionitrile was obtained, with a yield of 98.55%.
[0060] B, the synthesis of 2-cyanoethyl ethyl sulfonate:
[0061]
[0062] Add 2-hydroxypropionitrile (71.08g, 1mol), diisopropylethylamine (258.48g, 2mol) and 100mL toluene into a four-necked flask, cool down to 0-10°C, and slowly add ethylsulfonyl chloride dropwise (192.87g, 1.5mol), react at 0~10℃ for 24h after dropping. After filtering to remove salt, add 80 mL of saturated sodium carbonate solution to rinse the reaction solution, dry it with anhydrous magnesi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com