Zebrafish Nerve Tissue-Specific Enhancer and Its Cloning and Application
A neural tissue, specific technology, applied in the field of enhancer capture, can solve the problem of few specific enhancers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
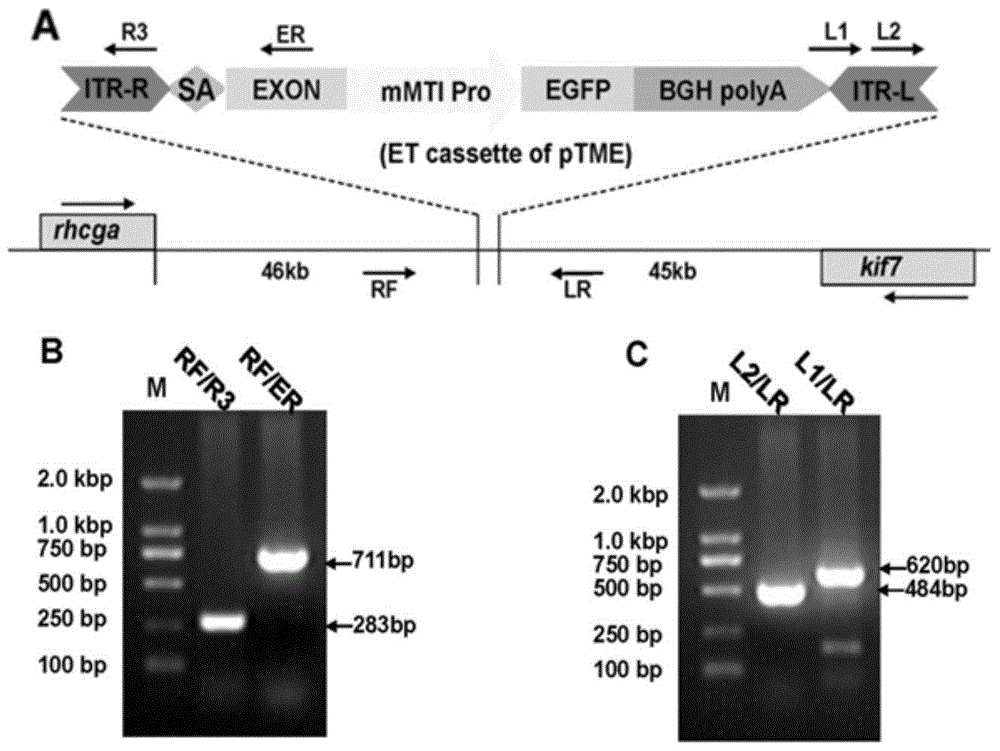
[0038] Example 1, construction of enhancer capture vector:
[0039] ① Design primers to amplify the mMT1 minimal promoter element from the pmMREd12mt1LUC3 plasmid, the required primer sequences:
[0040] Upstream primer: 5'-ATATTCGTACGACTCGTCCAACGACTATAA-3'(NO.3);
[0041] Downstream primer: 5'-CGGGGTACCGGTGAAGCTGGAGCTACG-3' (NO.4).
[0042] ②Using the pmMREd12mt1LUC3 plasmid as a template and using the above primers, a 105bp fragment was amplified. PCR cycle conditions: 94°C for 5min; 94°C for 30s, 60°C for 30s, 72°C for 30s, 30 cycles; 72°C for 10min, PCR amplified product It was recovered by 1% agarose gel electrophoresis kit.
[0043] ③ After the above PCR product was recovered and purified by gel, it was digested with restriction endonucleases BsiWI and KpnI, ligated with the pEF1ɑ-EGFP vector fragment that had undergone the same digestion, and transformed into Escherichia coli Top10'competent cells, and treated with ampicillin ( Amp)-LB plate screening to obtain posit...
Embodiment 2
[0049] Embodiment 2, zebrafish rearing and transgene microinjection
[0050] ① Breeding of zebrafish: zebrafish (Danio rerio), strain AB, feeding condition 28°C, 12h light / 12h night.
[0051] ② Synthesis of Tol2 transposase capped mRNA
[0052] A. Linearization: Use BamHI to digest the vector used for linearized transcription;
[0053] B. Use the BioFluxDNA Gel Recovery Kit to recover the linearized template, and dissolve the linearized template in DEPC water;
[0054] C. Transcription: T7 transcriptase (Ambion, Foster City, CA) in the mature mRNA synthesis kit was used for transcription. The transcription system is as follows:
[0055]
[0056] After mixing the above components, transcribe at 37°C for 2 hours;
[0057] D. After taking 1 μL for electrophoresis detection, add 0.5 μL 2U / μL DnaseI, and incubate at 37°C for 15 minutes;
[0058] E. Add 57.5 μL Nuclear-free water and 7.5 μL NH4AC to terminate the reaction;
[0059] F. Equal volume of phenol chloroform / chloro...
Embodiment 3
[0067] Example 3, Positive Screening of Transgenic Zebrafish
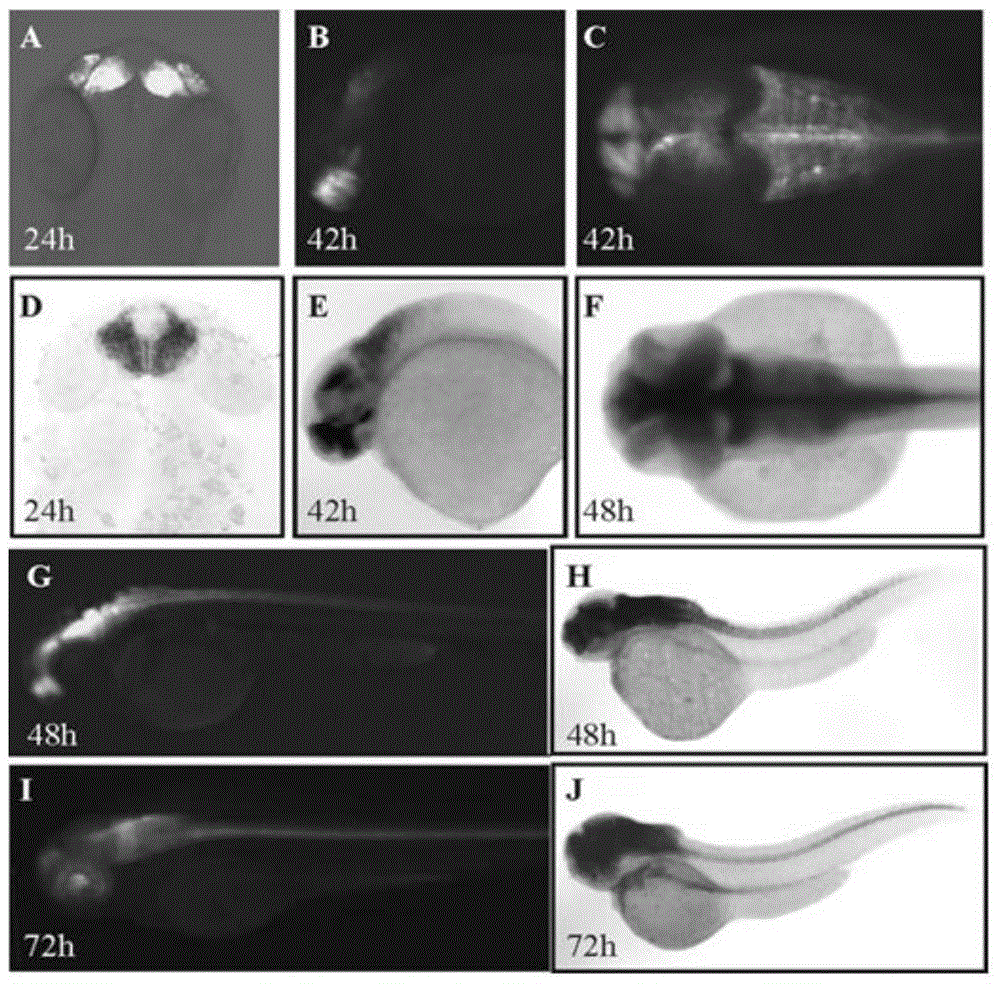
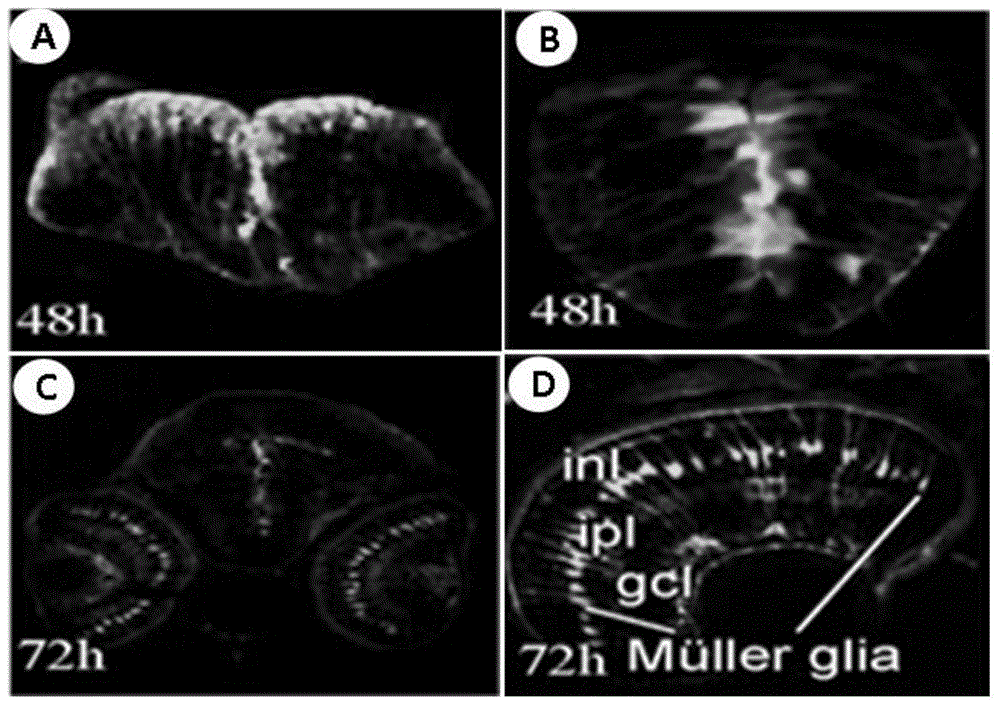
[0068] The sexually mature zebrafish of the F0 generation was crossed with the wild-type zebrafish, and the obtained F1 generation was observed with green fluorescence using a Carl Zeiss SteReo LumarV12 stereoscopic fluorescence microscope; the excitation wavelength of green fluorescent protein was 488nm, and the emission wavelength was 507nm, using Zeiss fluorescence inverted microscope was used to observe the fluorescence of F1 generation embryos at 12hpf, 24hpf, 48hpf, 72hpf, 4day, and 5day stages to obtain zebrafish embryos with specific green fluorescent protein expression in the nervous system; the fluorescent embryos were picked out and raised until Adult zebrafish are further crossed with wild-type zebrafish to obtain sufficient transgenic zebrafish heterozygous F2; heterozygous self-crossing can obtain fluorescently labeled homozygous F3.
[0069] Laser confocal microscopic observation and whole-mount in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com