Medicine of lansoprazole composition for treating digestive system diseases
A technology for digestive system diseases and lansoprazole, applied in the field of medicine, can solve problems such as affecting the quality of medicines and unsatisfactory results, and achieve the effects of good stability, excellent fluidity, and low content of insoluble particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
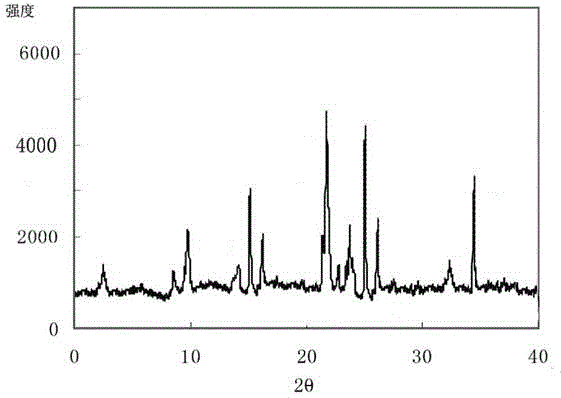
[0030] Example 1: Preparation of Lansoprazole Crystals
[0031] (1) Dissolving the crude product of lansoprazole into a mixed solution of methanol and carbon tetrachloride whose volume is 10 times the weight of lansoprazole at 35°C, the volume ratio of methanol and carbon tetrachloride is 4:2.5 ;
[0032] (2) adding activated carbon with a weight of 0.2 times the weight of lansoprazole for decolorization, and filtering to obtain a lansoprazole solution;
[0033] (3) Warm up the lansoprazole solution to 45°C, add distilled water dropwise to the lansoprazole solution under stirring conditions, the volume of distilled water is 8 times the weight of lansoprazole, and dropwise at a uniform speed within 0.5h, The stirring rate is 30rmp;
[0034] (4) After the dropwise addition, cool down to -15°C at a rate of 15°C / h, continue stirring at a stirring rate of 15rmp for 2h, let stand for 3h to precipitate crystals, filter, wash, and vacuum dry to obtain lansoprazole crystals.
[00...
Embodiment 2
[0036] Example 2: The preparation of lansoprazole composition
[0037] The composition is: 1 part by weight of the lansoprazole crystal prepared by the present invention, and 0.002 part by weight of sodium dihydrogen phosphate.
[0038] The preparation method is:
[0039] (1) Weigh lansoprazole crystals and sodium dihydrogen phosphate in proportion and mix them thoroughly;
[0040] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0041] Example 3: The preparation of lansoprazole composition
[0042] The composition is: 1 part by weight of the lansoprazole crystal prepared by the present invention, and 0.003 part by weight of sodium dihydrogen phosphate.
[0043] The preparation method is:
[0044] (1) Weigh lansoprazole crystals and sodium dihydrogen phosphate in proportion and mix them thoroughly;
[0045] (2) Dispense into sterilized vials and stopper them.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com