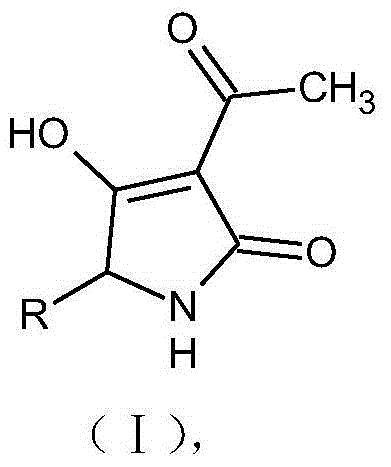
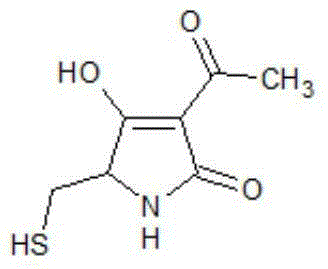
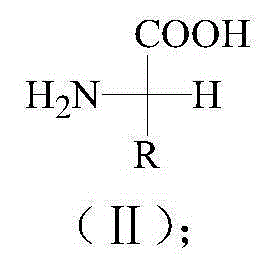Pyrrolidone sulfur derivatives and their preparation method and application
A technology of sulfur derivatives and pyrrolinone, which is applied in the field of sulfur derivatives of pyrrolinones and their preparation and application, and can solve the problems of easy loss of activity, spatial structure transformation, high cost and price of L-isoleucine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 3-acetyl-4-hydroxyl-5-mercaptomethyl-pyrroline-2-one (compound 1), its structural formula is:
[0040]
[0041] Its synthetic steps are:
[0042] Step 1. Preparation of L-cysteine methyl ester hydrochloride: Slowly add 0.12mol of thionyl chloride to 50mL of anhydrous methanol dropwise at -11°C. After stirring for 1 hour, add 0.1mol of L-cysteine After stirring at room temperature for 3 hours, stirring at 65°C for 4 hours, rotary evaporation, dissolving the reaction product in a mixed solvent of ethyl acetate and petroleum ether (volume ratio 1:1), crystallization was obtained at 0°C,
[0043] Step 2. Preparation of N-acetoacetylcysteine methyl ester: take 0.1 mol of the crystals obtained in step 1, dissolve in 50 mL of anhydrous methanol, add 0.1 mol of freshly prepared sodium methoxide to neutralize and remove hydrochloric acid, and then place in an ice-water bath at -10 °C 0.15mol diketene was added dropwise, stirred at room temperature for 16h, and 1M HCl and...
Embodiment 2
[0047] With reference to the preparation method of Compound 1 in Example 1, by adjusting the sulfur-containing amino acid as the starting material, the corresponding sulfur derivatives of pyrrolidones were synthesized, the reaction conditions and the ratio of raw materials used and the reaction conditions and raw materials used for preparing Compound 1 than the same.
[0048] Compound 2: Synthesis of 3-acetyl-4-hydroxy-5-(methyl-mercaptomethyl)-pyrroline-2 from 2-amino-3-(methyl-mercaptomethyl)propionic acid as starting material -ketone.
[0049] Compound 2: m.p.197.1-197.6°C. EI-MSm / z(%): 202[M+H] + , 155, 141(100), 98, 84, 48. 1 H-NMR (CDCl 3 , 500MHz), δ: 2.03 (t, J=7.0Hz, 3H, SCH 3 ), 2.51~2.54 (m, 2H, SCH 2 ), 2.34(s, 3H, COCH 3 ), 3.75 (d, J = 6.0 Hz, 1H, NCH), 7.63 (s, 1H, NH), 12.65 (s, 1H, OH).
[0050]
[0051] Compound 3: Synthesis of 3-acetyl-4-hydroxy-5-(ethyl-mercaptomethyl)-pyrroline-2 from 2-amino-3-(ethyl-mercaptomethyl)propionic acid as starting ma...
Embodiment 3
[0160] Take the above 38 sulfur derivatives of pyrrolinones (compound 1-6, compound 9-40), dissolve them with a small amount of methanol, and then prepare solutions with concentrations of 1 mg / L and 2 mg / L with distilled water, and set the same concentration Methanol solvent control and water control, Eupatorium adenophorum leaf acupuncture method for pathogenicity detection, each treatment repeated more than 6 times, 25 ° C, kept in natural light for 24 hours, and then measured the diameter of the lesion with a micrometer .
[0161] The results are shown in Table 1, indicating that sulfur derivatives of pyrrolinones have higher pathogenicity to Eupatorium adenophorum, and under the same concentration conditions, the longer the branch chain at the 5-position, the higher the pathogenicity.
[0162] Table 138 Pathogenicity data of sulfur derivatives of pyrrolinones to Eupatorium adenophorum
[0163]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com