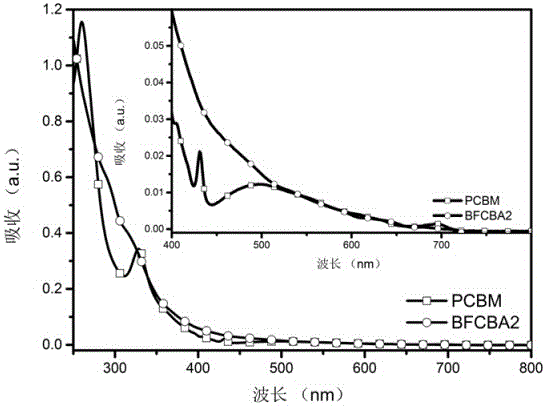
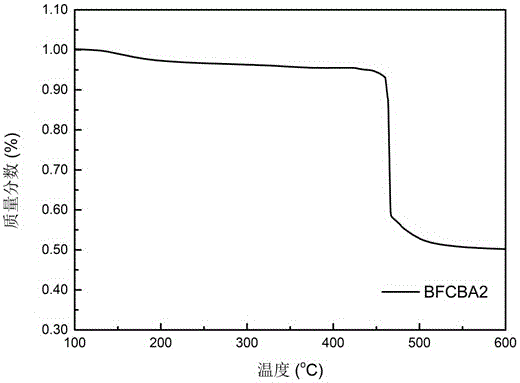
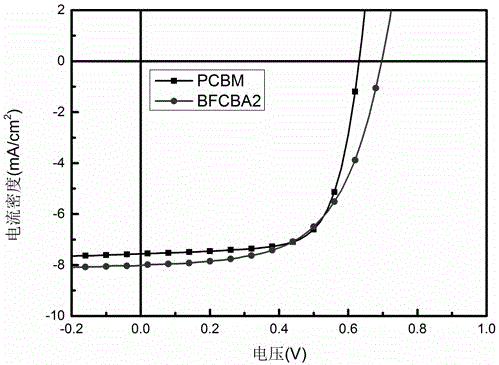
5-alkyl-2,3-dihydrobenzofuran-C60 fullerene bis-adduct and preparation method and application thereof
A double adduct, fullerene technology, applied in the field of material chemistry, can solve the problems of the arrangement affecting the mobility of carriers, reducing the effect, affecting the effect, etc., and achieving easy separation and purification, short reaction time, extremely high performance. The effect of scientific research and development and market investment value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: 5-isopropyl-2,3-dihydrobenzofuran-C 60 Preparation of fullerene bis-adducts (BFCBA1).
[0042] (1) In an argon atmosphere, add 2.80g (50mmol) KOH and 3.42g (20mmol) p-toluenesulfonamide to 80mL of anhydrous methanol, stir for 10min, then add 6.4g (20mmol) at 5°C ) Diacetoxyiodobenzene, stirred at 5°C for 0.5h, returned to room temperature and then reacted for 3h, poured the clear solution into the ice-water mixture to obtain a light yellow solid TsN=IPh crude product, filtered with a sand core The device was filtered, recrystallized with methanol, and vacuum-dried for 8 hours to obtain TsN=IPh, which was filled with nitrogen and stored in a refrigerator at 4°C until use. The yield was about 60%.
[0043]
[0044] (2) In an argon atmosphere, add 3.6mg (0.036mmol) of cuprous chloride and 6.0μL (0.072mmol) of 2,6-lutidine into the flask, and add it to 120mL of anhydrous o-dichlorobenzene, After stirring at room temperature for 30 min, 1.30 g (1.8 mmol) of C...
Embodiment 2
[0051] Example 2: 5-tert-butyl-2,3-dihydrobenzofuran-C 60 Preparation of fullerene bis-adducts (BFCBA2).
[0052] (1) In an argon atmosphere, add 2.80g (50mmol) KOH and 3.42g (20mmol) p-toluenesulfonamide to anhydrous methanol, stir for 10min, and o Add 6.4g (20mmol) diacetoxy iodobenzene at 5°C, stir for 0.5h at 5°C, return to room temperature and react for 3h, then pour the clear solution into ice-water mixture to obtain light yellow solid TsN=IPh crude product, filtered with a sand core filter, washed three times with ultrapure water and anhydrous ether, recrystallized with methanol, dried in vacuum for 8 hours to obtain TsN=IPh, filled with nitrogen and placed in a refrigerator at 4 °C Stored for later use, the yield is about 60%.
[0053] (2) In an argon atmosphere, add 3.6mg (0.036mmol) cuprous chloride, 6.0μL (0.072mmol) 2,6-lutidine into the flask, and then add 120mL of anhydrous o-dichlorobenzene, After stirring at room temperature for 30 min, 1.30 g (1.8 mmol) of ...
Embodiment 3
[0061] Example 3: 5-tert-amyl-2,3-dihydrobenzofuran-C 60 Preparation of fullerene bis-adducts (BFCBA3).
[0062] (1) In an argon atmosphere, add 2.80g (50mmol) KOH and 3.42g (20mmol) p-toluenesulfonamide to anhydrous methanol, stir for 10min, then add 6.4g (20mmol) at 5°C Diacetoxyiodobenzene, stirred at 5°C for 0.5h, returned to room temperature and then reacted for 3h, poured the clear solution into the ice-water mixture to obtain a light yellow solid TsN=IPh crude product, filtered through a sand core Filter, wash with ultrapure water and anhydrous ether for 3 times, recrystallize with methanol, and dry in vacuum for 8 hours to obtain TsN=IPh, fill with nitrogen and store in a refrigerator at 4°C for later use, the yield is about 60% .
[0063] (2) In an argon atmosphere, add 3.6mg (0.036mmol) cuprous chloride, 6.0μL (0.072mmol) 2,6-lutidine into the flask, and then add 120mL of anhydrous o-dichlorobenzene, After stirring at room temperature for 30 min, 1.30 g (1.8 mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com