Drug-loaded nanoparticle with reductive responsiveness and preparation method and applications thereof
A nano-drug-loaded, responsive technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve problems such as being pumped out of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Amphiphilic copolymer mPEG 45 -b-P(DssEEP 15 -EEP 20 )Synthesis
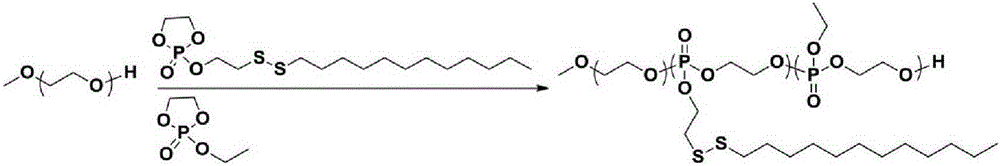
[0036] Polyethylene glycol-polyphosphate is composed of phosphate monomer EEP, DssEEP in the initiator PEG 2K Under the initiation of , TBD is obtained by ring-opening polymerization as a catalyst, and the synthetic route is as follows figure 1 shown.
[0037] 1. Preparation of phosphate monomer
[0038] First, 2-chloro-2-oxo-1,3,2-dioxaphospholane (2-chloro-2-oxo-1,3,2-dioxaphospholane, COP), then COP reacts with ethanol to synthesize EEP, and COP reacts with 2-hydroxyethyl dodecyl persulfide to synthesize DssEEP. Concrete synthetic method is as follows:
[0039] Synthesis of COP: In a 1000mL three-necked round-bottomed flask, dissolve phosphorus trichloride (3.0mol) in anhydrous dichloromethane (500mL), and slowly drop in ethylene glycol (3.0mol) through a constant pressure dropping funnel , after all the drops are finished, continue to react for 0.5 hours, evaporate the solvent under...
Embodiment 2
[0044] Example 2: Amphiphilic copolymer mPEG 45 -b-P(DssEEP 12 -EEP 6 )Synthesis
[0045] Polyethylene glycol-polyphosphate is composed of phosphate monomer EEP, DssEEP in the initiator PEG 2K Under the initiation of , TBD is obtained by ring-opening polymerization as a catalyst, and the synthetic route is as follows figure 1 shown.
[0046] 1, the preparation process of phosphate monomer is the same as embodiment 1.
[0047] 2. Synthesis of block polymers
[0048] The reaction was carried out in a glove box. The reaction was carried out in a glove box. Dissolve mPEG (100g, 0.05mol, Mn=2000g / mol), cyclic phosphate ester monomer DssEEP (0.6mol) and EEP (0.3mol) in THF (10L) (mPEG: DssEEP: EEP molar ratio is 1 :12:6), stir and mix evenly, add catalyst 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD, 0.1mol), react at 25°C for 3min, add benzoic acid ( 0.2mol) to terminate the reaction; the resulting product was precipitated twice in a mixed solvent of ether and methanol (10 / 1, ...
Embodiment 3
[0049] Embodiment 3: Characterization of amphiphilic copolymer PEG-b-P (EEP-DssEEP)
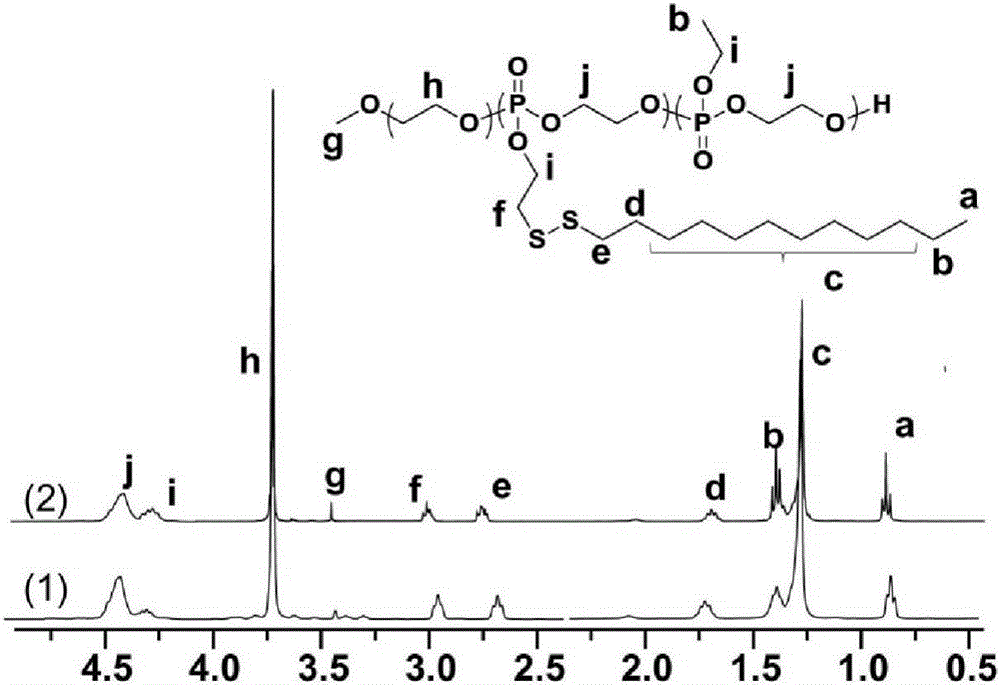
[0050] The polyethylene glycol-polyphosphate block copolymer that embodiment 1,2 prepares carries out proton nuclear magnetic resonance spectrum ( 1 HNMR) analysis to determine its molecular structure and link number, 1 HNMR spectrum see figure 2 .
[0051] figure 2 of polyethylene glycol-polyphosphate 1 The HNMR spectrum letters label the protic hydrogens assigned to the block polymer. Through the peak of 3.75ppm (assigned to the methylene group of polyethylene glycol), the peak of 4.31ppm (assigned to the methylene group of the PEEP side chain) and the peak of 2.96ppm (assigned to the methylene group of the PDssEEP side chain) The integral area ratio is calculated to obtain the number of phosphate linkages.
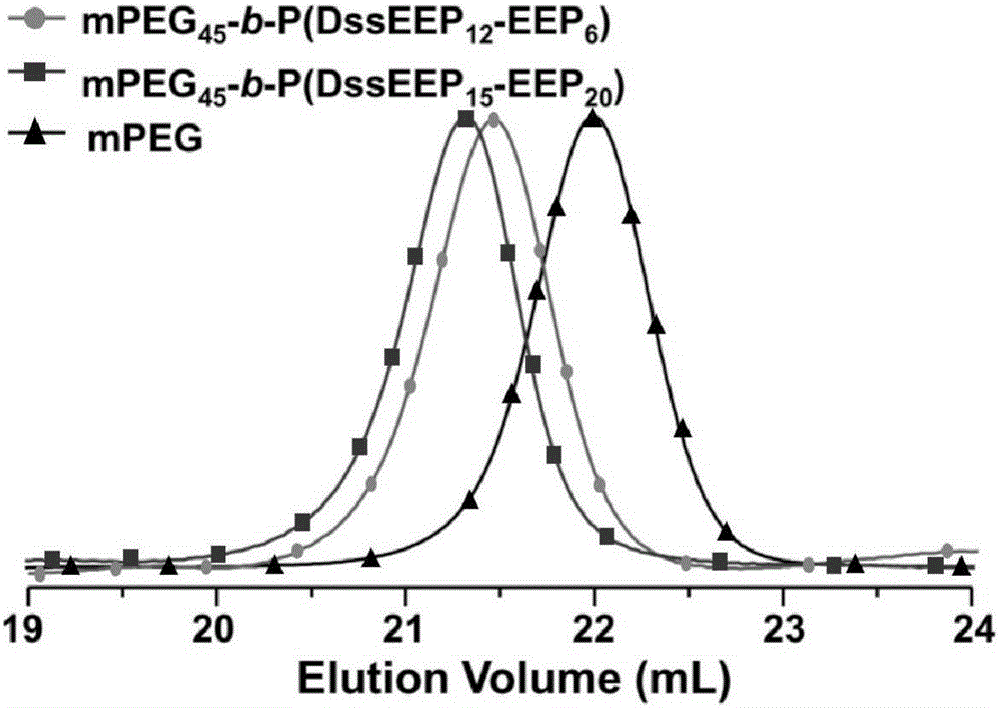
[0052] Gel Permeation Chromatography (GPC) method takes polyethylene glycol as the GPC collection of illustrative plates of the polyethylene glycol-polyphosphate block copolyme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com