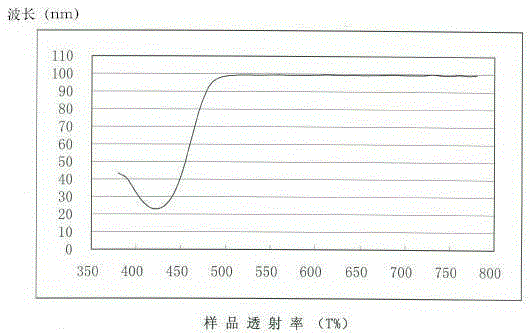
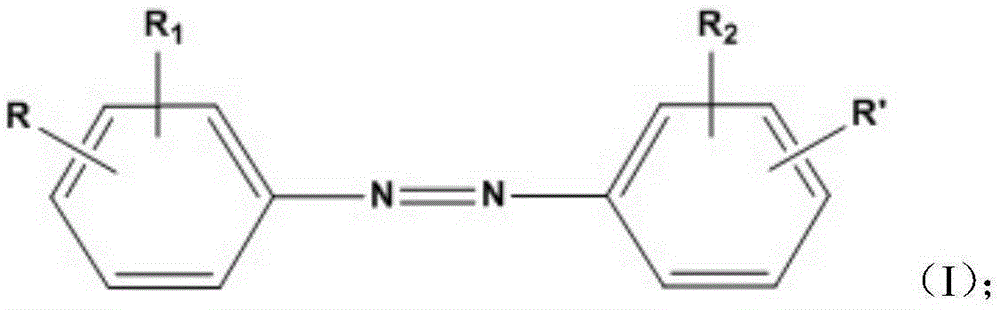
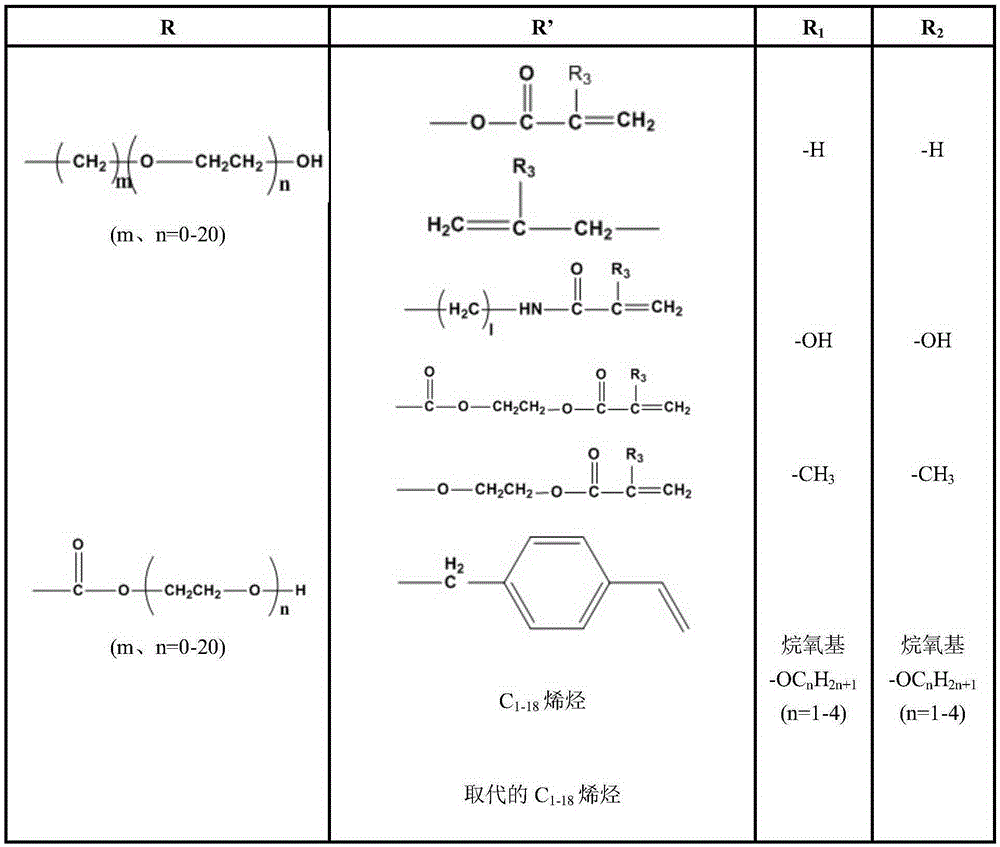
Blue light absorbent, blue-light prevention corneal contact lens containing blue light absorbent and manufacturing method of blue-light prevention corneal contact lens
A technology of contact lenses and blue light absorbers, applied in glasses/goggles, optics, optical components, etc., can solve problems such as loss, difficult biocompatibility evaluation of contact lenses, eye damage, etc., to avoid loss, improve Practicality, overcoming the effect of poor compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 contains PEG 200 Synthesis of Esterified and Acrylic Esterified Azo Blue Light Absorbers
[0034] Its structural formula is:
[0035]
[0036] The first step, the synthesis of diazonium salt
[0037] Under a nitrogen atmosphere, add 150mL of PEG200 / 1,4-dioxane / double distilled water (60 / 30 / 10) mixture into a 250mL three-necked flask, put it in an ice bath, and then add 5.23mL (0.0607 mol) concentrated hydrochloric acid, 4.156g (0.0303mol) carboxyaniline, then add NaNO dropwise 2 (2.30g, 0.0333mol) 10mL aqueous solution was stirred at 5°C for 1h, and a diazonium salt intermediate was formed.
[0038] The second step, the synthesis of azo compounds
[0039] Prepare 150 mL of PEG of phenol (8.5546 g, 0.0909 mol) and NaOH (1.33 g, 0.0333 mol) 500 / 1,4-dioxane / double distilled water (60 / 30 / 10) mixed solution, and then poured into the reacted first step diazonium salt solution, after the mixed solution was stirred for 15min, 350mL of water was added to adjust...
Embodiment 2
[0042] Example 2 contains PEG 200 Synthesis of Azo Blue Light Absorbers Esterified and Esterified with Hydroxyethyl Ethacrylate
[0043] Its structural formula is:
[0044]
[0045] The first step, the synthesis of azo compounds
[0046] Under nitrogen atmosphere, will contain carboxyaniline (0.2866g, 2.09mmol), NaNO 2 (0.19g, 2.85mmol), double distilled water (10mL) of concentrated hydrochloric acid (5mL) was added to tetrahydrofuran:pyridine (5:2) solution of benzoic acid (0.232g, 1.90mmol), stirred at 0°C for 15min, Then it was raised to room temperature, stirred for 48h, and the solvent was removed by rotary evaporation under reduced pressure. The obtained solid was dissolved in ethyl acetate (100mL), and the organic phase was washed with water (3*100mL). The combined organic layers were washed with anhydrous MgSO 4 After drying, the solvent was removed to obtain a red solid, which was recrystallized from dichloromethane-methanol to obtain a pure product.
[0047] T...
Embodiment 3
[0049] Example 3 contains PEG 200 Synthesis of Esterified and Acrylic Amidated Azo Blue Light Absorbers
[0050] Its structural formula is:
[0051]
[0052] The first step, the synthesis of amidated compounds
[0053] Add 2-phenylethylamine (3.96g, 0.0327mol) in 250mL three-necked flask to 100mL methanol solution, stir to make it dissolve completely, add methacrylic anhydride (5.089g, 0.033mol) dropwise in the reaction flask under stirring, After the dropwise addition, react at room temperature for 1 h, add 100 mL of 10% NaCl aqueous solution to it, then add 30 g of NaCl, filter out excess salt after stirring, and place the filtrate in the refrigerator overnight to obtain a white solid, which is mixed with 50:50 cold methanol : washed with water, the filtrate was placed in a refrigerator to obtain a white crystalline solid, combined solids, recrystallized with chloroform, filtered and dried in a vacuum oven to obtain amidated compounds.
[0054] The second step, the syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com