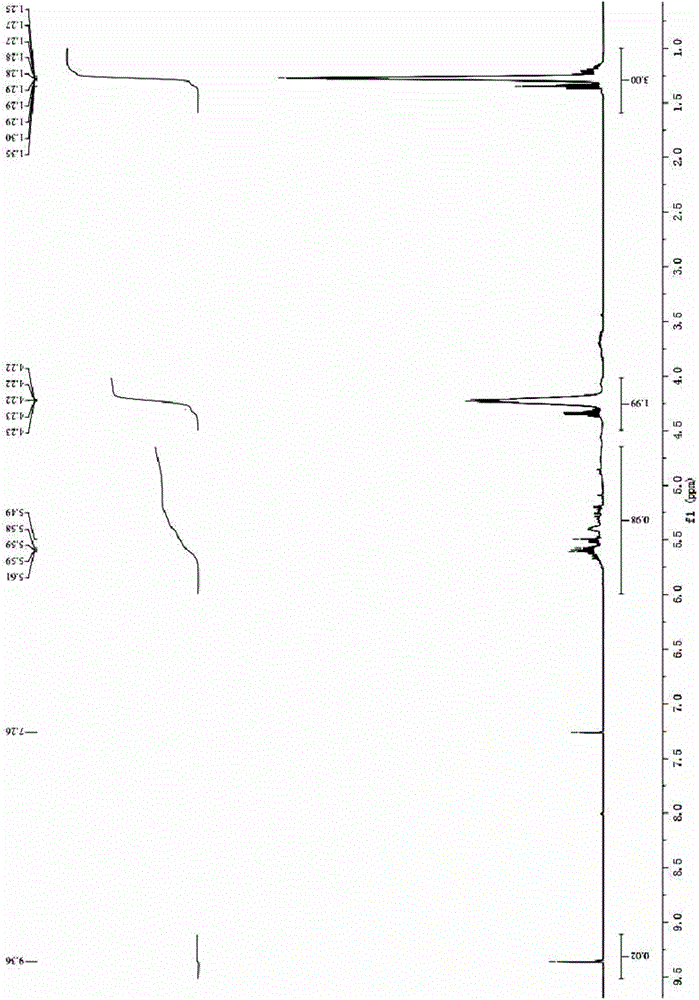
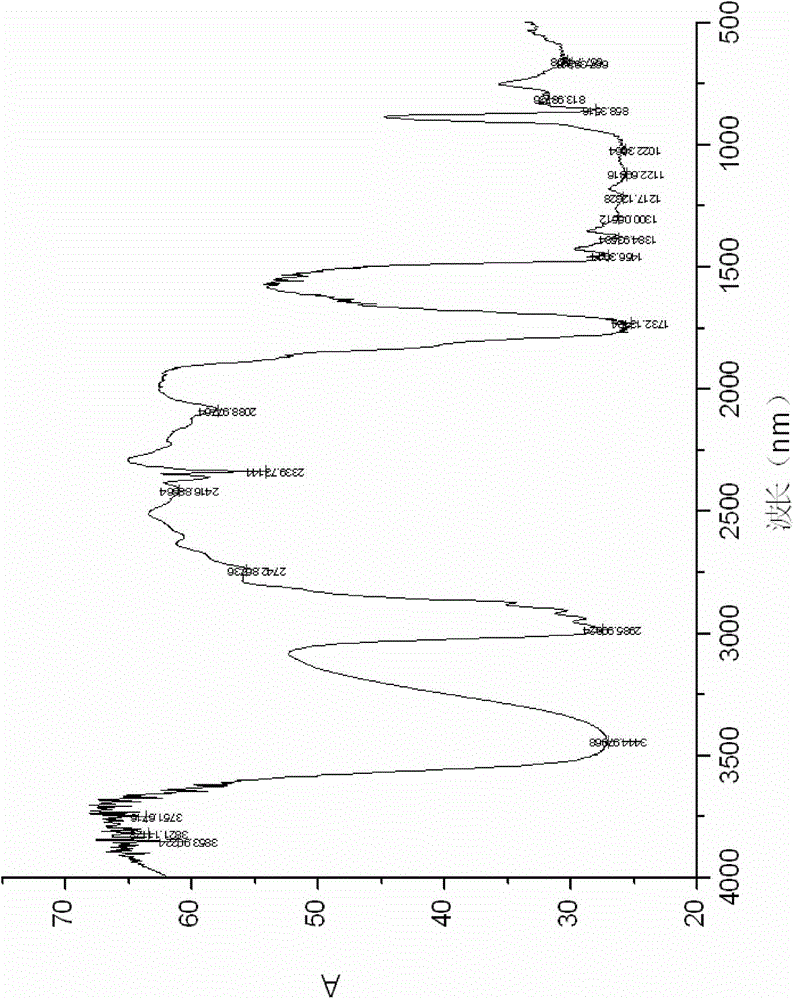
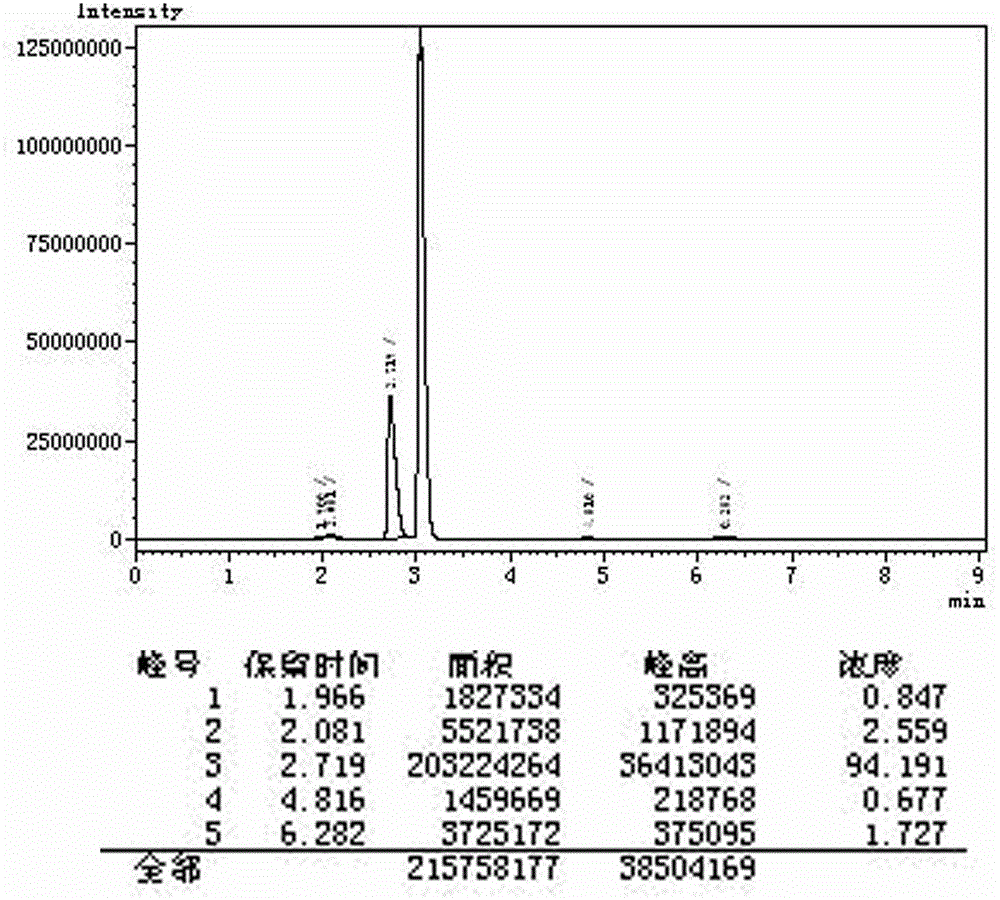
Synthetic method of glyoxylic ester
A synthesis method and glyoxylate technology, applied in the chemical industry, can solve the problems of serious environmental pollution, low content and low product content, and achieve the effects of simple reaction raw materials, avoiding environmental pollution and safe reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthesis of embodiment 1 ethyl glyoxylate
[0041] Add 740g (2mol) of 20% glyoxylic acid aqueous solution into the 1L reaction bottle, distill water under reduced pressure until no water is evaporated, and the glyoxylic acid dehydration is completed. After the concentrated glyoxylic acid in the reaction bottle is cooled, add ethanol 92g (2mol), 1.48g of p-toluenesulfonic acid (1% of the mass of glyoxylic acid), 74g of toluene (0.5 times of the mass of glyoxylic acid), put into the reaction bottle, open the cold heating mantle, and heat the reaction solution in the bottle to 60°C for reaction. During the reaction, divide water until no water comes out, and continue to distill to recover toluene. After the toluene has been distilled off, the temperature is lowered to below 25°C. Distill the above residual reaction solution under reduced pressure, maintain the vacuum of the system at 13-15mmHg, and receive the fraction at 65-68°C (theoretical value 64-66.5°C) to o...
Embodiment 2
[0042] The synthesis of embodiment 2 ethyl glyoxylate
[0043]Add 1480g (2mol) of 10% glyoxylic acid aqueous solution into the 1L reaction bottle, distill water under reduced pressure until no water is evaporated, and the glyoxylic acid dehydration is completed. After the concentrated glyoxylic acid in the reaction bottle is cooled, add ethanol 110.4g (2.4mol), 2.96g of hydrochloric acid (2% of the mass of glyoxylic acid), 148g of toluene (1 times the mass of glyoxylic acid), put into the reaction bottle, open the cold heating mantle, and heat the reaction solution in the bottle to 65°C, the reaction was carried out. During the reaction, divide water until no water comes out, and continue to distill to recover toluene. After the toluene has been distilled off, the temperature is lowered to below 25°C. Distill the above residual reaction solution under reduced pressure, maintain the vacuum of the system at 15mmHg, and receive the fraction at 65~68°C (theoretical value 64~66...
Embodiment 3
[0044] The synthesis of embodiment 3 ethyl glyoxylate
[0045] Add 184.1g (2.5mol) of solid glyoxylic acid into a 1L reaction flask, then add 128.8g (2.8mol) of ethanol, 5.92g of phosphoric acid (3% of the mass of glyoxylic acid), 222g of n-hexane (1.2% of the mass of glyoxylic acid) times), put it into the reaction bottle, turn on the cold heating mantle, heat the reaction solution in the bottle to reflux 70°C, and carry out the reaction. During the reaction process, divide water until no water comes out, and continue to distill and recover n-hexane. After the n-hexane has been distilled off, the temperature is lowered to below 25°C. Distill the above residual reaction solution under reduced pressure, maintain the vacuum of the system at 15mmHg, and receive the fraction at 65~68°C (theoretical value 64~66.5°C) to obtain the product. After vacuum distillation, 155.54 g of light yellow viscous liquid was obtained. The theoretical output is 204.18g, the product yield is 76....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com