Method for separating and measuring ezetimibe and relevant substances through high performance liquid chromatography
A technology of high performance liquid chromatography and ezetimibe, which is applied in the field of separation and determination of ezetimibe and related substances by high performance liquid chromatography, and can solve the problems of undetectable, degraded impurities, inability to separate, and incomplete separation and detection of impurities, etc. problems, to achieve precise quality control, accurate test results, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
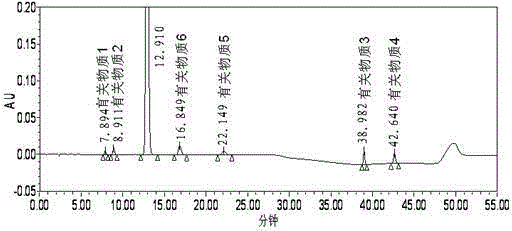
[0070] Embodiment 1 Determination of related substances of ezetimibe crude drug
[0071] Take appropriate amount of ezetimibe, related substance 1, related substance 2, related substance 3, related substance 4, related substance 5, and related substance 6, and use 0.1% phosphoric acid solution-acetonitrile-methanol (48:42:10) Dissolve and prepare a systemic suitability solution containing 200 μg of ezetimibe per 1 ml, 2 μg of each of related substance 1, related substance 2, related substance 3, related substance 4, related substance 5 and related substance 6.
[0072] Take ezetimibe reference substance, dissolve it with 0.1% phosphoric acid solution-acetonitrile-methanol (48:42:10) and prepare a reference substance solution containing 0.2μg per 1ml.
[0073] Take an appropriate amount of ezetimibe raw material, dissolve it with 0.1% phosphoric acid solution-acetonitrile-methanol (48:42:10), and prepare a test solution containing 200 μg of ezetimibe per 1 ml.
[0074] Take ...
Embodiment 2
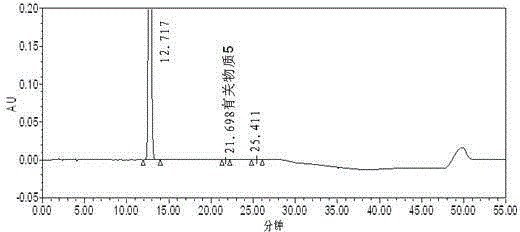
[0082] Example 2 Determination of Related Substances in Ezetimibe Preparations (Ezetimibe Tablets)
[0083] Take appropriate amount of ezetimibe, related substance 1, related substance 2, related substance 3, related substance 4, related substance 5, and related substance 6, and use 0.1% phosphoric acid solution-acetonitrile-methanol (48:42:10) Dissolve and prepare a systemic suitability solution containing 200 μg of ezetimibe per 1 ml, 2 μg of each of related substance 1, related substance 2, related substance 3, related substance 4, related substance 5 and related substance 6.
[0084] Take ezetimibe reference substance, dissolve it with 0.1% phosphoric acid solution-acetonitrile-methanol (48:42:10) and prepare a reference substance solution containing 0.4μg per 1ml.
[0085] Grind ezetimibe tablets finely, take an appropriate amount, and use 0.1% phosphoric acid solution-acetonitrile-methanol (48:42:10) to sonicate for 30 minutes (shake at any time), and prepare a supply ...
Embodiment 3
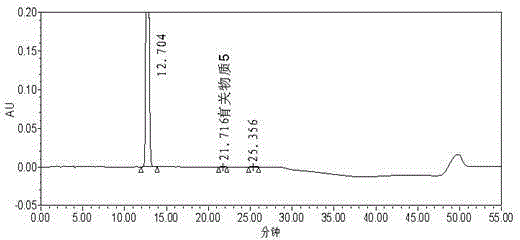
[0105] Example 3 Detection of related substances after ezetimibe alkali degradation
[0106] Chromatographic column: phenylsilane bonded silica gel as filler (PhenomenexLunaPhenylHexyl250mm×4.6mm, 5μm);
[0107] Mobile phase: 0.1% phosphoric acid solution: acetonitrile: methanol (48:42:10);
[0108] Detection wavelength: 232nm;
[0109] Injection volume: 30μl;
[0110] Detection time: 25 min (isocratic elution part of the method of the present invention).
[0111] Take an appropriate amount of ezetimibe, put it in a measuring bottle, add 10ml of 0.01mol / L ethanol-made sodium hydroxide solution to dissolve, seal it tightly, heat at 55°C for 15 minutes, take it out, immediately add 2ml of 0.1mol / L hydrochloric acid solution, and use 0.1% phosphoric acid solution-acetonitrile-methanol (48:42:10) is dissolved, is mixed with the need testing solution that every 1ml contains ezetimibe 200 μ g, gets this need testing solution and injects high performance liquid chromatograph, ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com