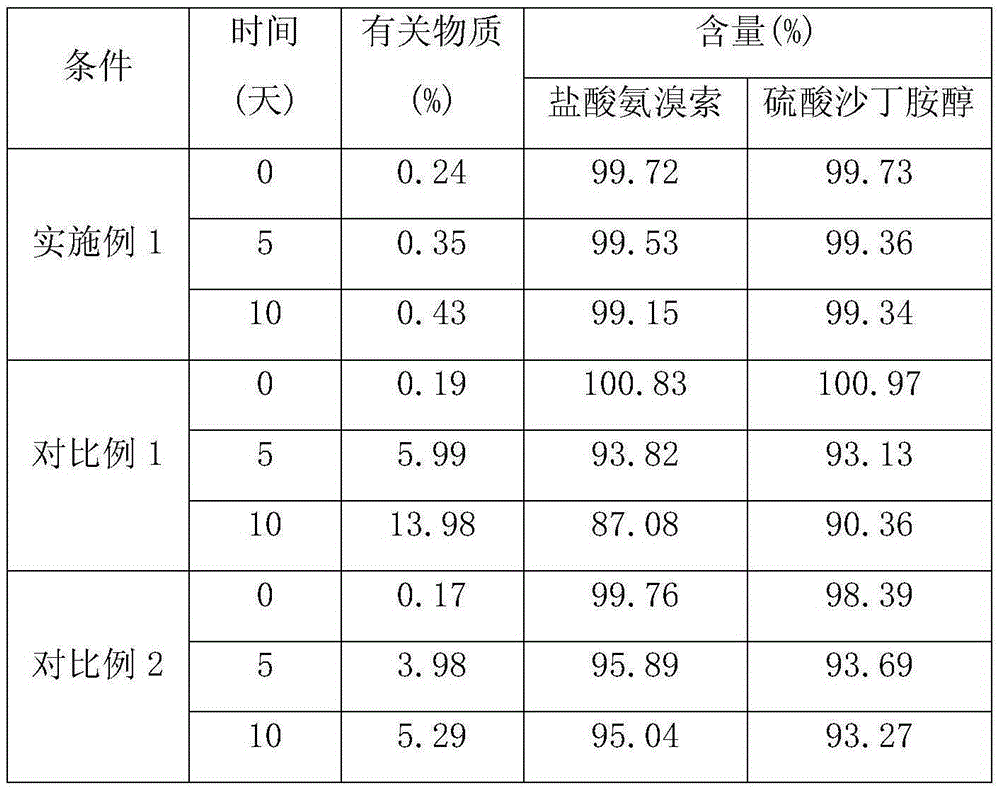
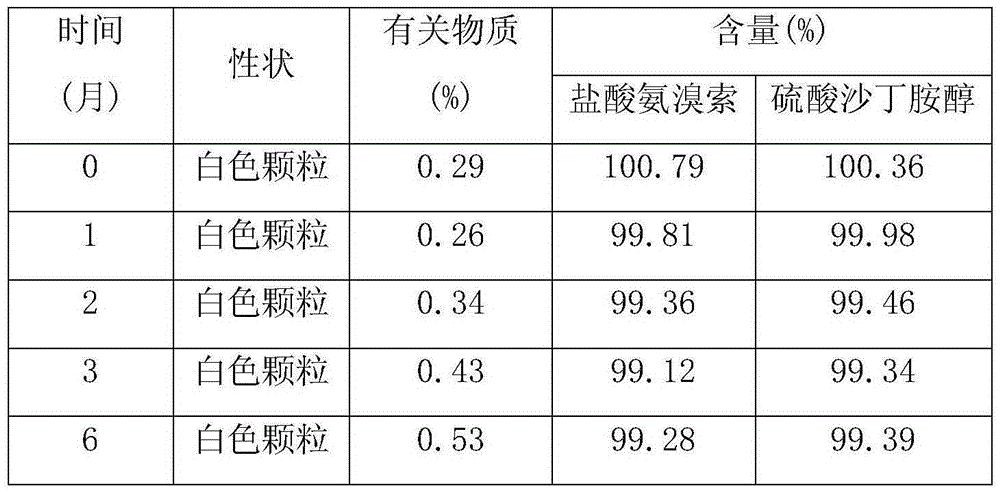
Ambroxol salbutamol enteric particles
An ambroxoxalbutamol and enteric-coated technology, which is applied to the medical preparations of non-active ingredients, respiratory diseases, organic active ingredients, etc., can solve the problem of unsatisfactory product stability, no reports of enteric-coated particles, and easy side effects. Products and other problems, to achieve the effect of increasing the content of active ingredients, treating respiratory diseases, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] prescription:
[0050] The core is made of the following components by weight:
[0051] Salbutamol sulfate: 12g;
[0052] Ambroxol hydrochloride: 15g;
[0053] Poloxamer 188: 8g;
[0054] Meglumine: 12g;
[0055] Mannitol: 18g;
[0056] Lactose: 60g;
[0057] Sodium carboxymethyl starch: 5g;
[0058] Povidone: Appropriate amount.
[0059] The enteric coating layer is made of the following components by weight: 15 g of cellulose acetate phthalate.
[0060] Preparation:
[0061] 1) Weigh the raw and auxiliary materials of the prescription amount, pass through a 100-mesh sieve for subsequent use;
[0062] 2) Add distilled water to the sieved povidone and cellulose acetate phthalate respectively to make a binder solution and a coating solution;
[0063] 3) Mix the sieved salbutamol sulfate, ambroxol hydrochloride, poloxamer 188, meglumine, mannitol, lactose and carboxymethyl starch sodium in equal increments, and add the above adhesive solution to prepare into soft...
Embodiment 2
[0067] prescription:
[0068] Core:
[0069] Salbutamol sulfate: 10g;
[0070] Ambroxol hydrochloride: 15g;
[0071] Poloxamer 188: 5g;
[0072] Meglumine: 15g;
[0073] Mannitol: 15g;
[0074] Dextrin: 80g;
[0075] Microcrystalline cellulose: 2g;
[0076] Hypromellose: Appropriate amount.
[0077] Coating layer: 10 g of cellulose acetate trimellitate.
[0078] Preparation:
[0079] 1) Weigh the raw and auxiliary materials of the prescription amount, pass through a 100-mesh sieve for subsequent use;
[0080] 2) Take the sieved hypromellose and cellulose acetate trimellitate and add distilled water respectively to make a binder solution and a coating solution;
[0081] 3) Mix the sieved salbutamol sulfate, ambroxol hydrochloride, poloxamer 188, meglumine, mannitol, dextrin and microcrystalline cellulose in equal increments, and add the above adhesive solution to prepare into soft material;
[0082] 4) After granulating with a 40-mesh sieve, dry at 60°C for 30 minut...
Embodiment 3
[0085] prescription:
[0086] Core:
[0087] Salbutamol sulfate: 15g;
[0088] Ambroxol hydrochloride: 20g;
[0089] Poloxamer 188: 10g;
[0090] Meglumine: 12g;
[0091] Mannitol: 20g;
[0092] Starch: 60g;
[0093] Microcrystalline cellulose: 4g;
[0094] Povidone: Appropriate amount.
[0095] Coating layer: 20 g of hydroxypropyl cellulose phthalate.
[0096] Preparation:
[0097] 1) Weigh the raw and auxiliary materials of the prescription amount, pass through a 100-mesh sieve for subsequent use;
[0098] 2) Take povidone and hydroxypropyl cellulose phthalate after sieving and add distilled water respectively to make binder solution and coating solution;
[0099] 3) Mix the sieved albuterol sulfate, ambroxol hydrochloride, poloxamer 188, meglumine, mannitol, starch and microcrystalline cellulose in equal increments, and add the above binder solution to prepare soft material;
[0100] 4) After granulating with a 40-mesh sieve, dry at 60°C for 60 minutes, pass thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com