Method for manufacturing cyclic carbonate
一种制造方法、碳原子数的技术,应用在化学仪器和方法、物理/化学过程催化剂、有机化合物/氢化物/配位配合物催化剂等方向,能够解决不能得到充分满足、经时收率降低、催化剂量减少等问题,达到抑制催化剂更新成本、高转化率、有利制造的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
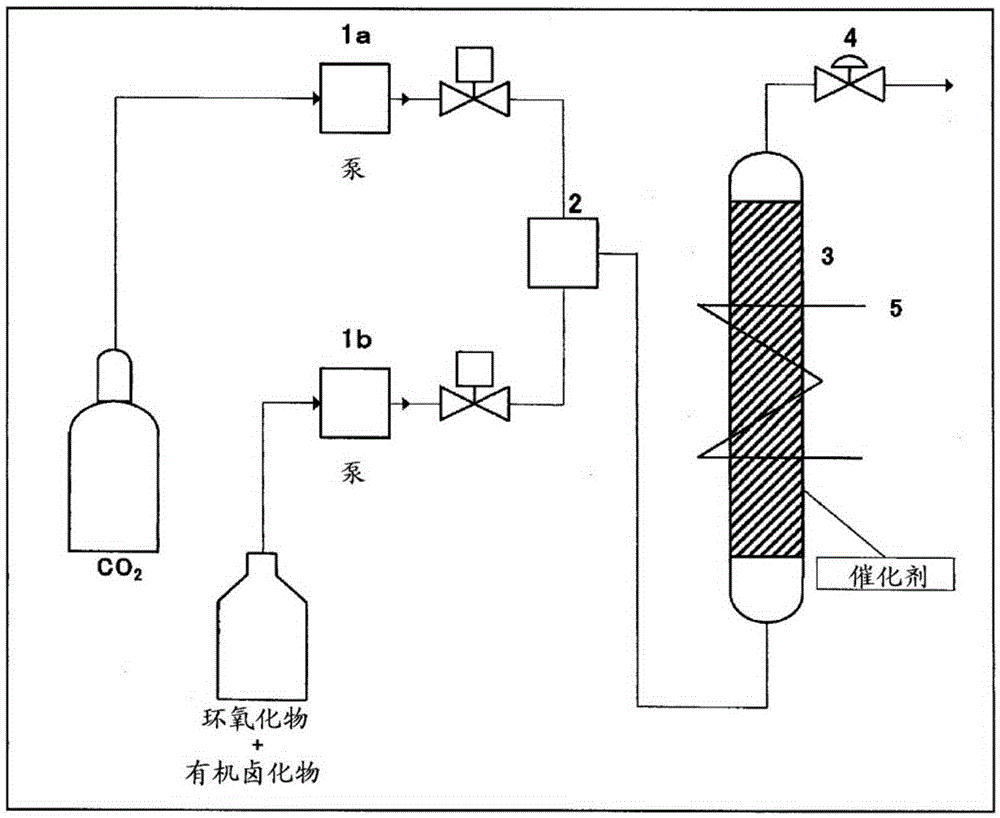
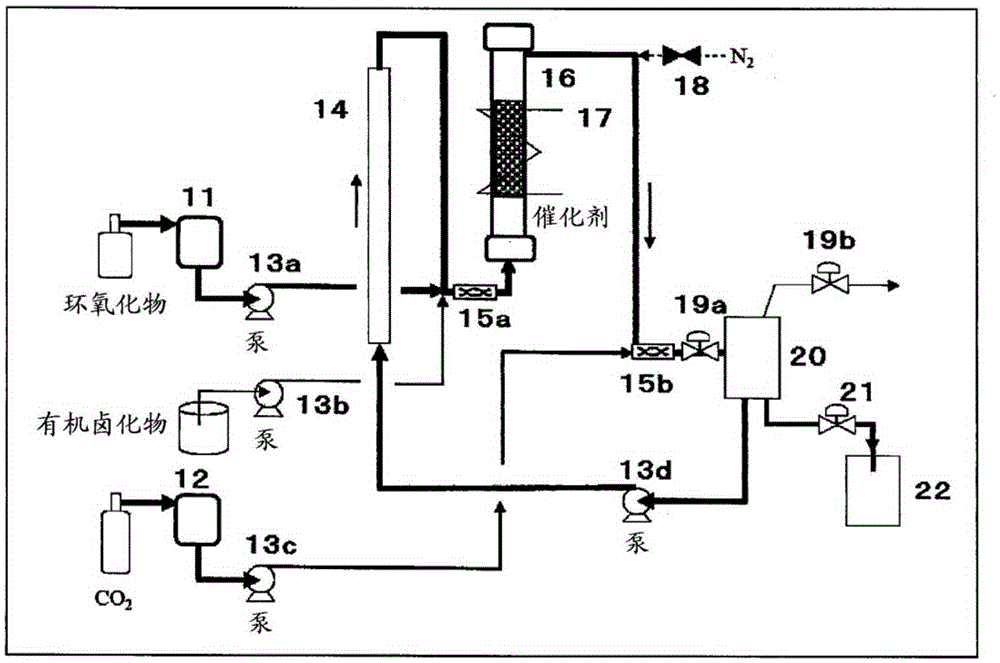
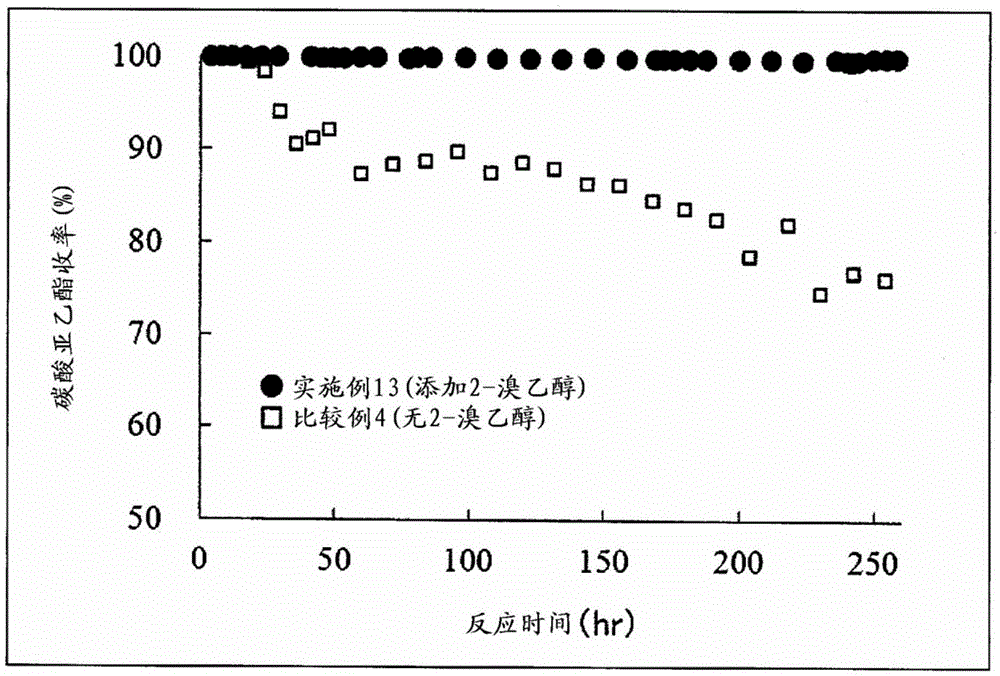
Image
Examples
Embodiment
[0112] Hereinafter, although an Example is given and this invention is demonstrated in detail, this invention is not limited to these Examples.
[0113] The analysis methods used in the respective examples and comparative examples are as follows.
[0114] (1) Fluorescence X-ray analysis
[0115] The amount of bromine, chlorine, and phosphorus modification of the catalyst was measured using fluorescent X-ray analysis (device: product name "System 3270" (manufactured by Rigaku Denki Kogyo Co., Ltd.), measurement conditions: Rh tube bulb, tube voltage 50kV, tube current 50mV, vacuum environment, Detector: SC, F-PC).
[0116] (2) Gas chromatography analysis
[0117] Compositional analysis of the reaction solution and the like used gas chromatography. The analysis conditions are as follows.
[0118] Device: Product name "GC-2010Plus" (manufactured by Shimadzu Corporation)
[0119] Detector: FID
[0120] INJ temperature: 150°C
[0121] DET temperature: 260°C
[0122] Sample ...
Synthetic example 1
[0126] Catalyst Synthesis Example 1: Tributyl Bromide Synthesis of Surface Modified Silica Catalyst (Catalyst A)
[0127] Bead silica gel (CARiACTQ-10 manufactured by Fuji Silysia Chemical (average pore diameter 10nm, particle diameter 1.2-2.4mm, specific surface area 300m 2 / g)) 20g and 50mL of 2N hydrochloric acid were put into a 200mL three-necked flask with stirring blades, and the inside of the flask was replaced with nitrogen and heated to reflux for 4 hours, thereby demetallizing the silica gel. Thereafter, the silica gel was separated by filtration, and sufficiently washed with ion-exchanged water. In addition, when 1N silver nitrate aqueous solution was dripped at the liquid after washing|cleaning, it did not become cloudy, and it was confirmed that it does not contain chlorine and that washing|cleaning was sufficient.
[0128] Put the acid-treated silica gel and 50 mL of toluene into a 200 mL three-neck flask with stirring blades equipped with Dean-Stark, and perf...
Synthetic example 2
[0132] Catalyst Synthesis Example 2: Tributyl Bromination Synthesis of Surface Modified Silica Catalyst (Catalyst B)
[0133] Bead silica gel (CARiACTQ-10 manufactured by Fuji Silysia Chemical (average pore diameter 10nm, particle diameter 1.2-2.4mm, specific surface area 300m 2 / g)) 2000g and 5000mL of xylene were put into a 10L three-necked flask with stirring blades equipped with a Dean-Stark separator, and azeotropic dehydration of xylene-water was carried out at 140°C for 2 hours to remove the moisture in the silica gel . Next, the Dean-Stark separator was removed, and after replacing the inside of the flask with nitrogen, 219 g (0.846 mol) of 3-bromopropyltrimethoxysilane was added dropwise. The silanization reaction was carried out by directly heating it to reflux at 135° C. for 7 hours. Next, the obtained reactant was separated by filtration, washed twice with xylene, and 3810 g of a catalyst precursor (bromopropylated silica gel) containing xylene was obtained. N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aperture size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com