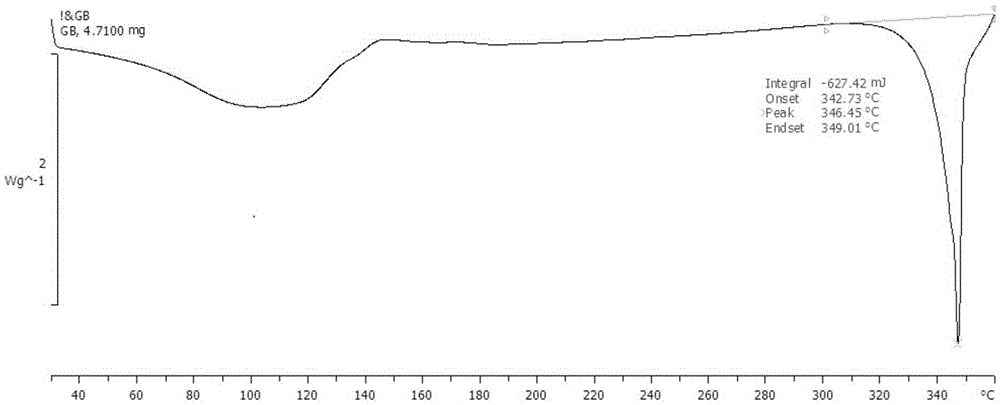
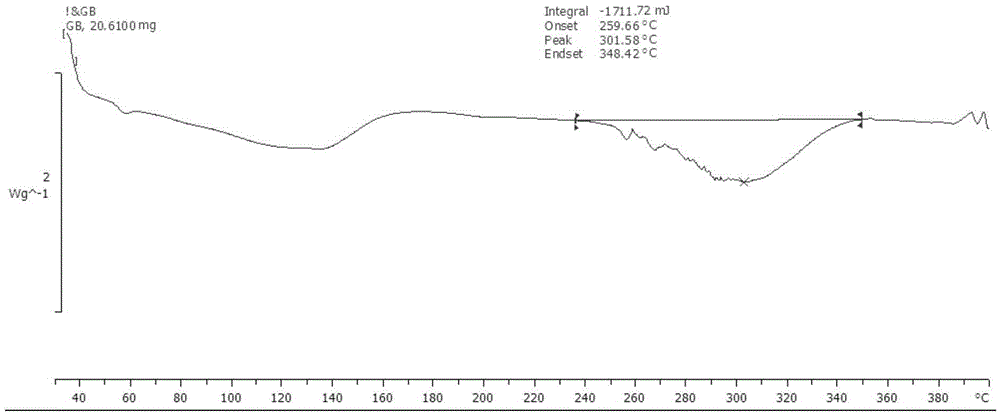
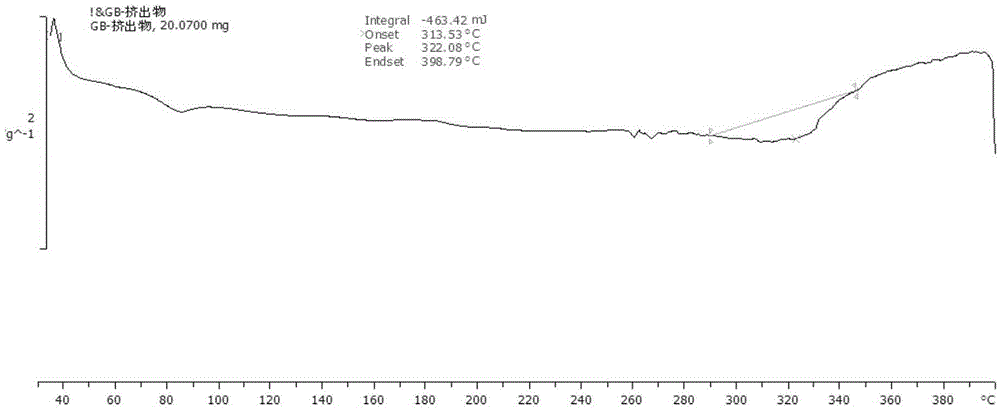
Ginkgolide B amorphous solid dispersion prepared through hot-melt extrusion method
A solid dispersion and ginkgolide technology, which is applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of ginkgolide crystal state characterization, poor clinical curative effect, Problems such as low drug loading, to achieve the effect of improving drug solubility and dissolution rate, improving release rate and release uniformity, high solubility and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Dry grinding micronization treatment of ginkgolide B
[0042] Weigh the ginkgolide B, carrier material and plasticizer under different prescriptions in Table 1 and mix them evenly, then add them to the dry grinding equipment, add grinding beads with a diameter of 12mm about 30% of the grinding cylinder, and grind beads with a diameter of 5mm about Grinding 10% of the cylinder, sealing the grinder, setting the speed at 50r / min, room temperature, and grinding for different times. The specific prescription grinding time is shown in Table 2 to prepare micronized ginkgolide B carrier eutectic powder.
[0043] Table 1 Composition of different prescriptions
[0044]
[0045]
[0046] Table 2 Grinding time of different prescriptions and powder particle size after grinding
[0047] 1) Determination of drug particle size after dry grinding and micronization:
[0048] The particle size of the milled product was checked by a laser particle size analyzer, and the r...
Embodiment 2
[0057] Embodiment 2 Ginkgolide B solid dispersion preparation
[0058] Get 200g of ginkgolide B drug-loaded eutectic powder of prescription 1 in Example 1 through micronization, add in the feeding hopper of the hot-melt extruder; After the stability, turn on the oil pump and turn on the main machine and feed at the same time to prepare a solid dispersion; collect the extruded product into a stainless steel pan, cool at room temperature for 24 hours, pulverize, and pass through a 20-80 mesh sieve to obtain ginkgolide B solid dispersion particles or powder .
Embodiment 3
[0059] Embodiment 3 Ginkgolide B solid dispersion preparation
[0060] Take prescription 2 in Example 1 and 200 g of ginkgolide B drug-loaded eutectic powder that has been micronized, and add it to the feeding hopper of the hot-melt extruder; set the host frequency to 3.5 Hz, the feeding frequency to 3 Hz, and the temperature to 170 ° C; After the temperature is stable, turn on the oil pump and turn on the main machine and the feed at the same time to prepare a solid dispersion; collect the extruded product into a stainless steel pan, cool at room temperature for 24 hours, pulverize, and pass through a 20-80 mesh sieve to obtain ginkgolide B solid dispersion particles or powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com