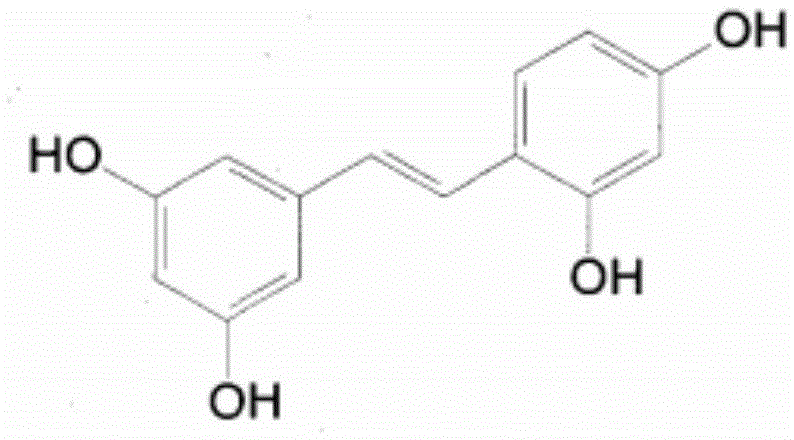
Oxyresveratrol synthesis method
A technique for oxidizing resveratrol and a synthetic method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of harsh reaction conditions, low yield, expensive raw materials, etc., and achieve low cost and high yield. The effect of high efficiency and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1, 3, the preparation of 5-dimethoxybenzyl chloride
[0020] Put 42g of 3,5 dimethoxybenzyl alcohol and 126ml of phosphorus oxychloride into a dry 500ml four-necked reaction flask, heat up and reflux for about 2 hours, TLC detection, the raw materials are basically reacted, pressurize and recover the solvent to 1 / 3 and cool down To 0-5°C, slowly add 150ml of water dropwise, after the addition is complete, stir at room temperature for 2h, filter, wash with water to neutralize, and dry under reduced pressure to obtain 45g of white solid with a yield of 96.7%.
[0021] 2. Preparation of tetramethoxystilbene
[0022] In a dry 500ml four-necked reaction flask, put 37.2g of 3,5-dimethoxybenzyl chloride, 27g of trimethyl phosphite, and 80ml of DMF, heat up and reflux for 4.5h, TLC detection, raw material 3,5-dimethoxy Benzyl chloride disappeared. Cool down to 5-10°C, add 29.8g of 28% sodium methoxide solution dropwise, keep stirring at this temperature for 2h after dropping,...
Embodiment 2
[0026] 1, 3, the preparation of 5-dimethoxybenzyl chloride
[0027] Put 42g of 3,5 dimethoxybenzyl alcohol and 150ml of phosphorus oxychloride into a dry 500ml four-necked reaction flask, heat up and reflux for about 2 hours, TLC detection, the raw materials are basically reacted, pressurize and recover the solvent to 1 / 3 and cool down To 0-5°C, slowly add 180ml of water dropwise, after the addition is complete, stir at room temperature for 2h, filter, wash with water to neutralize, and dry under reduced pressure to obtain 42.5g of white solid with a yield of 91.3%.
[0028] 2. Preparation of tetramethoxystilbene
[0029] In a dry 500ml four-necked reaction flask, put 37.2g of 3,5-dimethoxybenzyl chloride, 26g of trimethyl phosphite, and 120ml of DMF, heat up and reflux for 4 hours, TLC detection, raw material 3,5-dimethoxychloride Benzyl disappears. Cool down to 5-10°C, add 30g of 28% sodium methoxide solution dropwise, keep stirring at this temperature for 2h after droppin...
Embodiment 3
[0033] 1, 3, the preparation of 5-dimethoxybenzyl chloride
[0034] Put 42g of 3,5 dimethoxybenzyl alcohol and 175ml of phosphorus oxychloride into a dry 500ml four-necked reaction flask, heat up and reflux for about 4 hours, TLC detection, the raw materials are basically reacted, pressurize and recover the solvent to 1 / 3 and cool down To 0-5°C, slowly add 200ml of water dropwise, after the addition is complete, stir at room temperature for 2h, filter, wash with water to neutralize, and dry under reduced pressure to obtain 43.0g of white solid with a yield of 93.6%.
[0035] 2. Preparation of tetramethoxystilbene
[0036]Into a dry 500ml four-necked reaction flask, put 37.2g of 3,5-dimethoxybenzyl chloride, 35g of trimethyl phosphite, 90ml of DMF, heat up and reflux for 2.5h, TLC detection, raw material 3,5-dimethoxy Benzyl chloride disappeared. Cool down to 5-10°C, add 44g of 28% sodium methoxide solution dropwise, keep stirring at this temperature for 2h after dropping, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com