Macrolide antimicrobial compound as well as preparation method and application thereof
A technology of macrolides and compounds, applied in the field of macrolides antibacterial compounds and their preparation, achieving good application prospects, solving the problem of multi-drug resistance, and good antibacterial performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
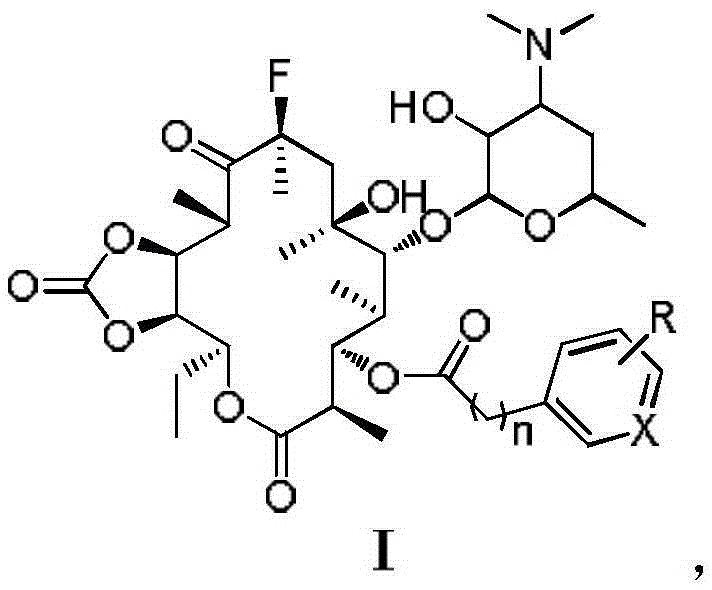
[0033] The present embodiment macrolide antibacterial compound I 1 Synthesis:
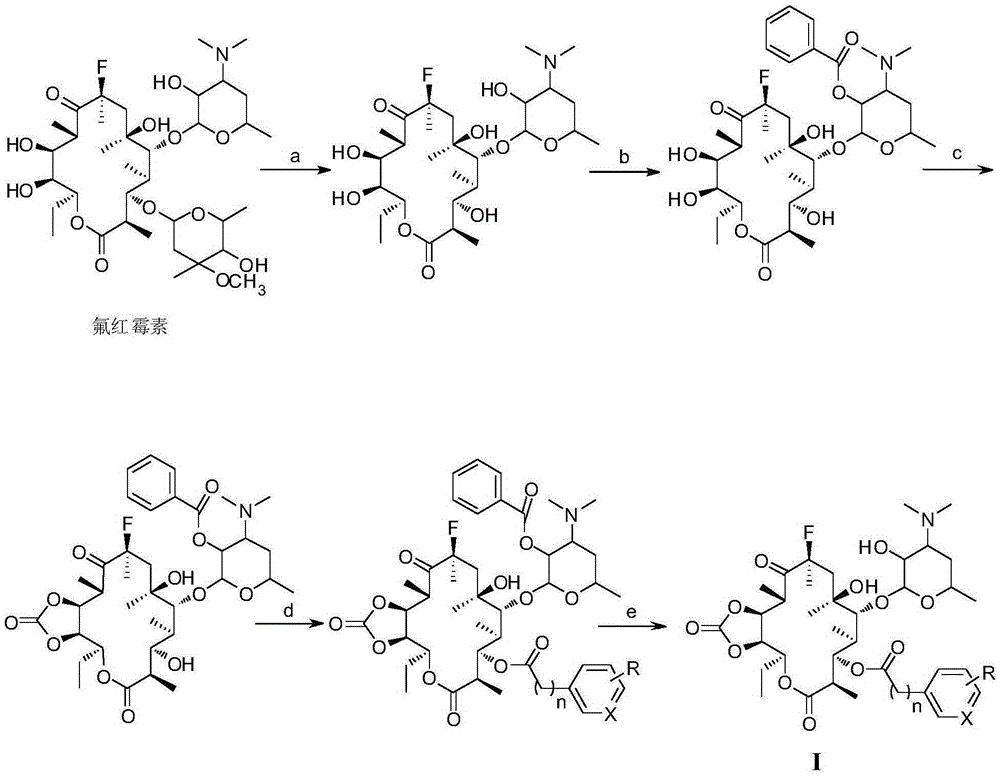
[0034] (1) Fluoromycin (70.0 g, 93 mmol) was dissolved in 1200 mL of hydrochloric acid buffer solution with a pH of 1.5, and the reaction was stirred at room temperature for 24 h. The solution was concentrated to 200 mL, and the pH was adjusted to 9 by adding ammonia water. Ethyl acetate was extracted three times (150 mL×3), and the organic phase was successively washed with saturated sodium bicarbonate solution (100 mL×3) and saturated saline solution (100 mL×3), and dried overnight over anhydrous sodium sulfate. Filtration, evaporation of solvent, silica gel column chromatography (developing agent V 二氯甲烷 / V 甲醇 =20:1), the white solid product 3-de(hexylglucopyranosyl)-3-hydroxyfluoroerythromycin (40.0g, 72%) was obtained, and the product identification data were as follows:
[0035] m.p.:118-120℃; 1 H-NMR (400MHz, DMSO-d 6 )δ: 5.11(d, J=7.8Hz, 2H, H-1', 13), 4.48(d, J=7.3Hz, 1H, H-6-OH), 4.1...
Embodiment 2
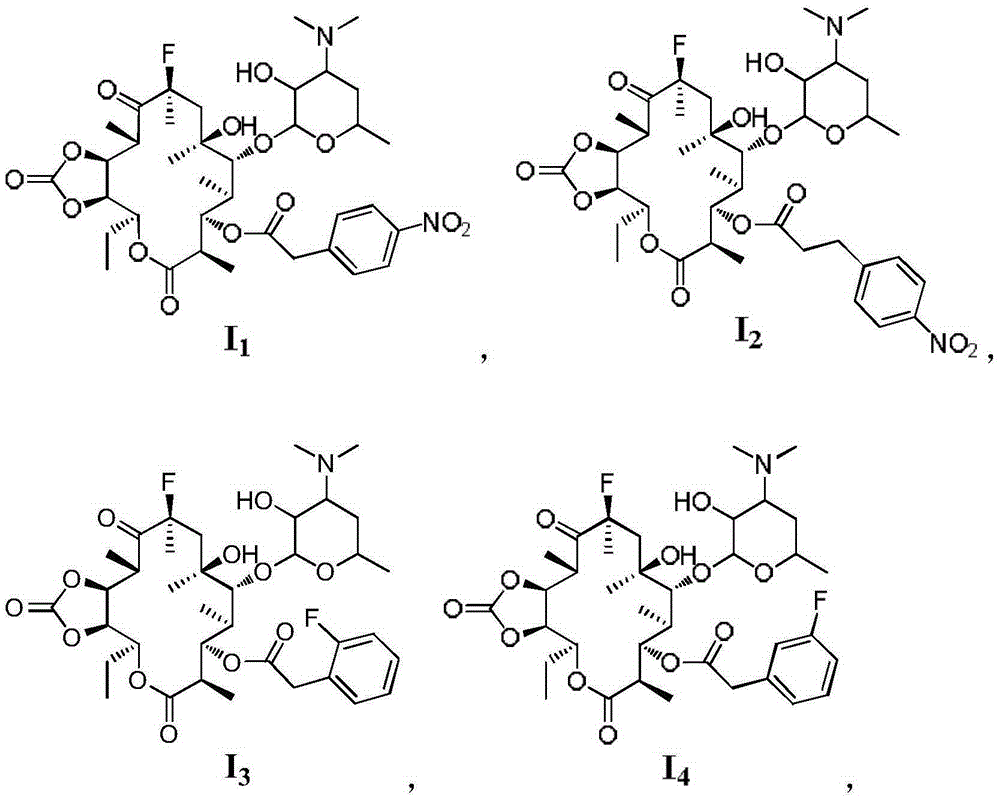
[0044] The present embodiment macrolide antibacterial compound I 2 Synthetic, the preparation method is the same as in Example 1, p-nitrophenylacetic acid (0.31g, 1.73mmol) is replaced by p-nitrophenylpropionic acid (0.34g, 1.73mmol), and the light yellow solid product 3-de(hexylpyridine) is obtained Glucopyranosyl)-3-p-nitrophenylpropionyl-11,12-cyclocarbonate-fluerythromycin I 2 (0.4g, 36%) product identification data are as follows:
[0045] m.p.:110-113℃; 1 H-NMR (400MHz, CDCl 3 )δ: 8.13(d, J=8.1Hz, 2H, H-2”, 6”), 7.40(d, J=8.2Hz, 2H, H-3”, 5”), 5.81(d, J=10.7 Hz,1H,H-1'),4.94(d,J=9.2Hz,1H,H-13),4.82(s,1H,H-11),4.22(d,J=7.0Hz,1H,H- 3), 3.84(dt, J=13.2, 6.6Hz, 2H, H-7”-CH 2 -),3.45(s,1H,H-5'),3.27-3.07(m,2H,8”-CH 2 -),2.73(ddd,J=23.8,16.4,9.2Hz,4H,H-2,2',10,5,),2.48(d,J=31.8Hz,6H,H-3'-N-( CH 3 ) 2 ), 2.09(q, J=6.5Hz, 1H, H-3'), 1.71(s, 3H, H-6-CH 3 ), 1.61 (d, J=23.1Hz, 4H, H-4'b, 4, 7a, 13- CH 2a CH 3 ),1.48(s,3H,H-8-CH 3 ), 1.19 (d, J=7.2Hz, 3H, H-4'a, 7b, ...
Embodiment 3
[0047] The present embodiment macrolide antibacterial compound I 3 The synthesis of, preparation method is the same as embodiment 1, p-nitrophenylacetic acid (0.31g, 1.73mmol) is replaced by 2-fluorophenylacetic acid (0.27g, 1.73mmol), silica gel column chromatography (developing agent V 二氯甲烷 / V 甲醇 =40:1), the white solid product 3-de(hexyglucopyranosyl)-3-(2-fluoro-phenylacetyl)-11,12-cyclocarbonate-fluerythromycin I was obtained 3 (0.25g, 24%), the product identification data are as follows:
[0048] m.p.:90-92℃; 1 H-NMR (400MHz, DMSO-d 6 )δ:7.68-7.65(m,2H,H-2",6"),7.50(d,J=7.8Hz,2H,H-3",5"),5.86(s,1H,H-1' ),5.59(dd,J=10.0,1.4Hz,1H,H-13),4.88(dd,J=9.8,3.3Hz,1H,H-2'-OH),4.60(s,1H,H-11 ), 4.31(d, J=4.0Hz, 1H, H-3), 4.10(d, J=7.4Hz, 1H, H-6-OH), 3.84(t, J=8.8Hz, 3H, H-7 "-CH 2 -,5'),3.48(dd,J=10.1,5.1Hz,1H,H-5),3.13(dd,J=7.3,1.6Hz,1H,H-2'),3.05-2.98(m,1H ,H-7a),2.72(dd,J=26.1,13.7Hz,2H,H-10,2),2.21(s,6H,H-3'-N-(CH 3 ) 2 ), 1.99(d, J=1.8Hz, 1H, H-7b), 1.96(d, J=6.8Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com