A fusion protein with functions of anti-tumor, anti-inflammation and treatment of ophthalmic diseases and its preparation method and application
A fusion protein, ophthalmic disease technology, applied in antitumor drugs, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve the problems of short half-life, high chemical synthesis cost, single target, etc., and achieve low toxicity , Improve efficacy, enhance the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Construction of the carrier
[0047] The full lengths of the target genes of the two fusion proteins are 195bp and 174bp respectively, the plasmid vector is pGEX-4T-1, the cloning site is BamHI / XhoI, and the host bacteria are DH5α or CHO cells. Among them, GGATCC is a BamHI restriction site, CTCGAG is an XhoI restriction site, and TAGTAA is two stop codons.
[0048] Target gene base sequence:
[0049] The base sequence of protein A gene is:
[0050] 5' GGATCC GCATGCGATTGCCGTGGTGATTGCTTTTGCGGTGGTGGTGGTATCGTGCGCCGTGCCGACCGCGCAGCCGTGCCCGCGGAAGCCGCGGCGAAAGAAGCCGCGGCGAAAGAAGCCGCGGCGAAAGAAGCCGCGGCGAAAGCGATCGTGCGCCGTGCCGACCGCGCAGCCGTGCCCGGTGGTGGTGGTCGTGGTGAT TAGTAACTCGAG 3'
[0051] The gene base sequence of protein G is:
[0052] 5' GGATCC GCATGCGATTGCCGTGGTGATTGCTTTTGCGGTGGTGGTGGTATCGTGCGCCGTGCCGACCGCGCAGCCGTGCCCGGTGGTGGTGGTTCTGGTGGTGGTGGTTCTGGTGGTGGTGGTTCTATCGTGCGCCGTGCCGACCGCGCAGCCGTGCCCGGTGGTGGTGGTCGTGGTGAT TAGTAACTCGAG 3'
[0053] (2) Expression of the tar...
Embodiment 2
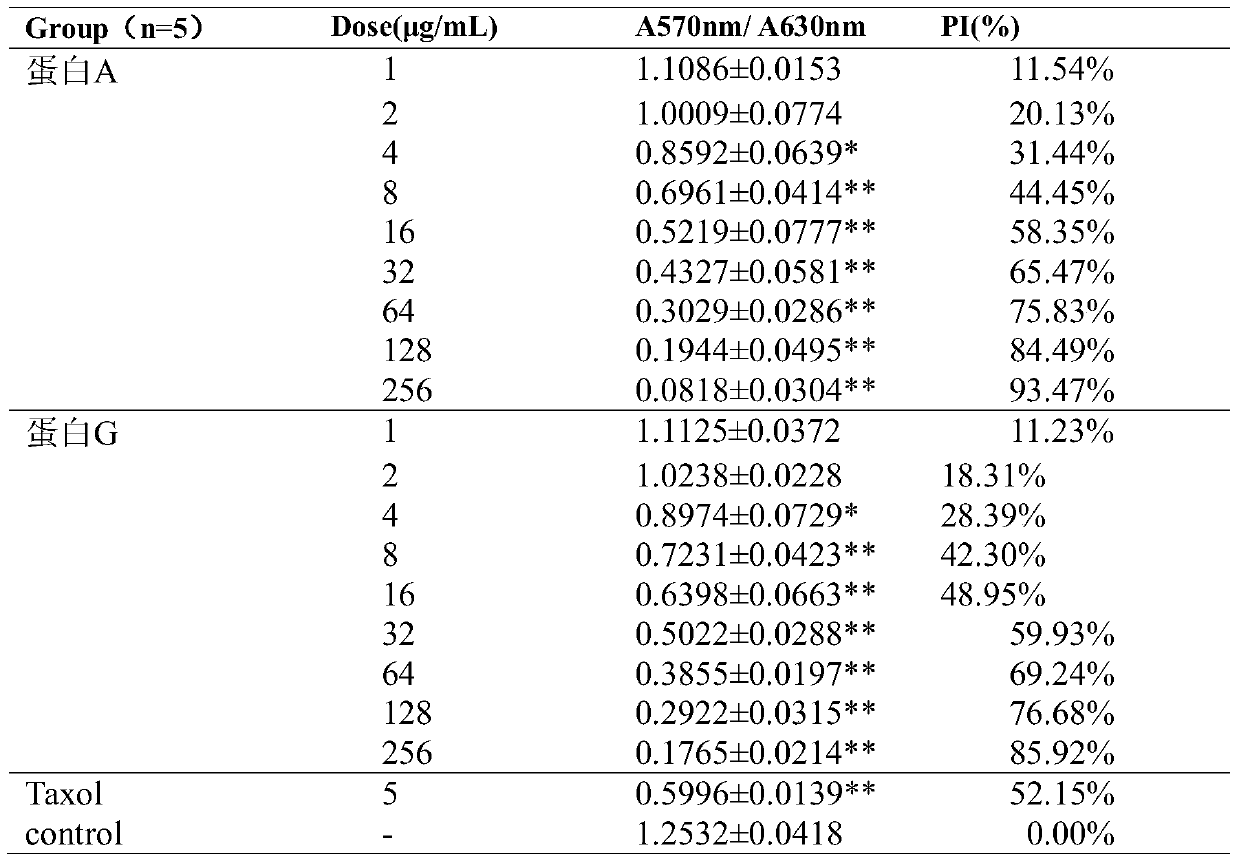
[0081] Inhibitory Effects of Fusion Proteins on the Proliferation of Various Tumor Cells
[0082] MTT method was used to detect the inhibitory effect of the integrin blocker fusion protein obtained in Example 1 on the proliferation of various tumor cells, including melanoma cell B16F10, gastric cancer cell MGC-803, lung cancer cell A549, liver cancer cell Hep-G2, Breast cancer cell MDA-MB-231, colon cancer cell HCT-116, human glioma U87, cervical cancer cell Hela.
[0083] Tumor cells were incubated at 37°C, 5% CO 2 When cultured in an incubator with a density of more than 90%, it was digested with trypsin and collected, and the cells were resuspended in culture medium and counted under a microscope, and the cell concentration was adjusted to 3.0×10 4 cells / mL, seed the cell suspension into a 96-well plate, 100 μL per well, and inoculate at 37°C, 5% CO 2 Incubate overnight in the incubator. Dilute fusion protein A, fusion protein G, and positive drug Taxol to respective pre...
Embodiment 3
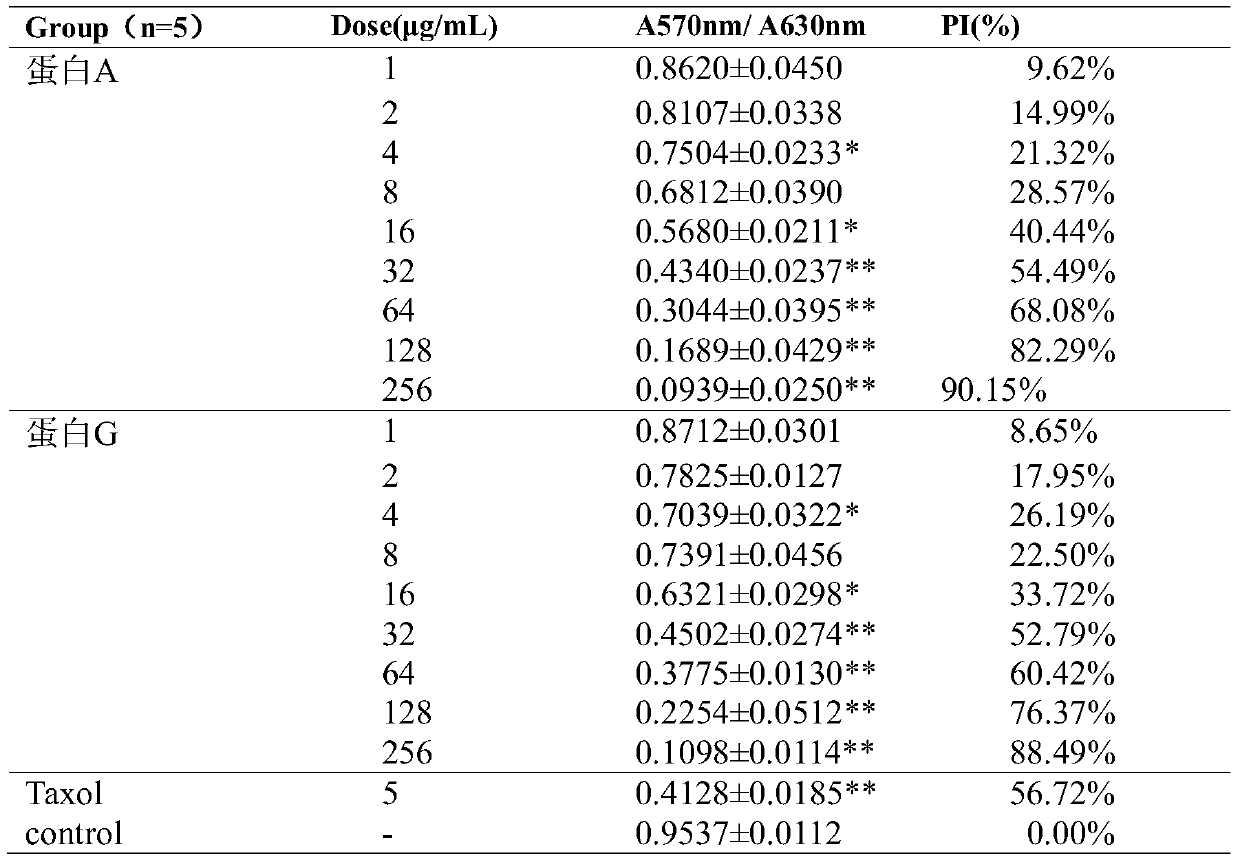
[0124] Three-dimensional transwell method to detect the activity of fusion protein A and protein G in inhibiting the migration of human umbilical vein endothelial cells
[0125] Human umbilical vein endothelial cells (HUVEC) were incubated with endothelial cell culture medium containing 5% fetal bovine serum and 1×ECGS at 37°C, 5% CO 2 When cultured in the incubator to a confluence of more than 90%, the transwell method was used to detect the activity of fusion protein A and protein G in inhibiting the migration of endothelial cells. The endothelial cell HUVEC only used the 2nd to 8th passages. The specific operation is as follows:
[0126] (1) Dilute 10mg / mL Matrigel with DMEM medium at a rate of 1:4, spread on the transwell chamber membrane, and air-dry at room temperature;
[0127] (2) Digest the HUVEC cells cultivated to the logarithmic growth phase with 0.2% EDTA, collect, wash twice with PBS, resuspend with endothelial cell culture medium containing 0.1% BSA, count under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com