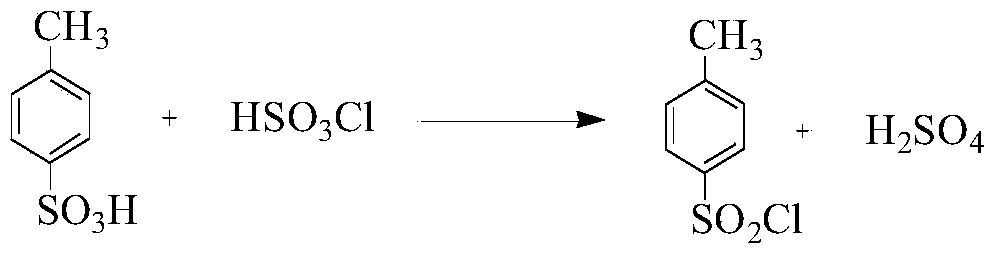
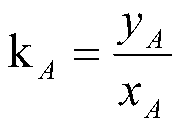Preparation method of p-toluene sulfonyl chloride
A technology of p-toluenesulfonyl chloride and p-toluenesulfonic acid is applied in the preparation of sulfonic acid, bulk chemical production, organic chemistry, etc., and can solve the problems of three wastes, large amount of chlorosulfonic acid, and unfavorable large-scale industrial production. The effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 0.1mol (19.02g) of p-toluenesulfonic acid (with crystal water, the same below) and 0.38g [BuPy]EtOSO 3 Dissolve in 47.55g of chloroform, stir evenly, and add 0.15mol (17.48g) of chlorosulfonic acid dropwise at 25°C. After constant temperature reaction for 2.5 h, 0.1 mol (1.8 g) of distilled water was added to remove excess chlorosulfonic acid. The reaction solution was separated into layers, and the waste acid and the organic phase were obtained by liquid separation through a separatory funnel. 10 g of distilled water was added to the organic phase for washing to remove residual p-toluenesulfonic acid in the organic phase, and the waste water and the product layer were obtained by liquid separation. The distribution coefficient of p-toluenesulfonic acid is the ratio of the concentration of p-toluenesulfonic acid in the organic phase to the concentration in the spent acid. Analysis by liquid chromatography showed that the distribution coefficient of p-toluenesulfonic ac...
Embodiment 2
[0029] 0.1mol (19.02g) of p-toluenesulfonic acid and 0.19g [Bmim] EtOSO 3 Dissolve in 47.55g of chloroform, stir evenly, and add 0.1mol (11.65g) of chlorosulfonic acid dropwise at 25°C. After the addition, 0.05 mol (0.9 g) of distilled water was added after constant temperature reaction for 3 h to remove excess chlorosulfonic acid. The reaction solution was separated into layers, and the waste acid and the organic phase were obtained by liquid separation through a separatory funnel. 10 g of distilled water was added to the organic phase for washing, the residual p-toluenesulfonic acid in the organic phase was extracted, and the waste water and the product layer were obtained by liquid separation. The distribution coefficient of p-toluenesulfonic acid is the ratio of the concentration of p-toluenesulfonic acid in the product layer to the concentration in the waste acid. Analysis by liquid chromatography showed that the distribution coefficient of p-toluenesulfonic acid was 0.0...
Embodiment 3
[0031] 0.1mol (19.02g) of p-toluenesulfonic acid and 0.57g [Bmin] BF 4 Dissolve in 19.02g of chloroform, stir evenly, and add 0.15mol (17.4g) of chlorosulfonic acid dropwise at 25°C. After the addition, 0.1 mol (1.8 g) of distilled water was added after constant temperature reaction for 2 h to remove excess chlorosulfonic acid. The reaction solution was separated into layers, and the waste acid and the organic phase were obtained by liquid separation through a separatory funnel. 10 g of distilled water was added to the organic phase for washing, the residual p-toluenesulfonic acid in the organic phase was extracted, and the waste water and the product layer were obtained by liquid separation. The distribution coefficient of p-toluenesulfonic acid is the ratio of the concentration of p-toluenesulfonic acid in the product layer to the concentration in the waste acid. Analysis by liquid chromatography showed that the distribution coefficient of p-toluenesulfonic acid was 0.0371;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com