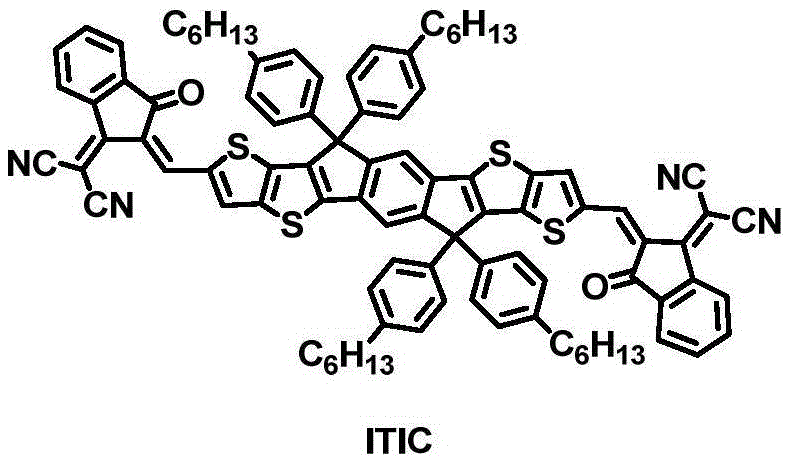
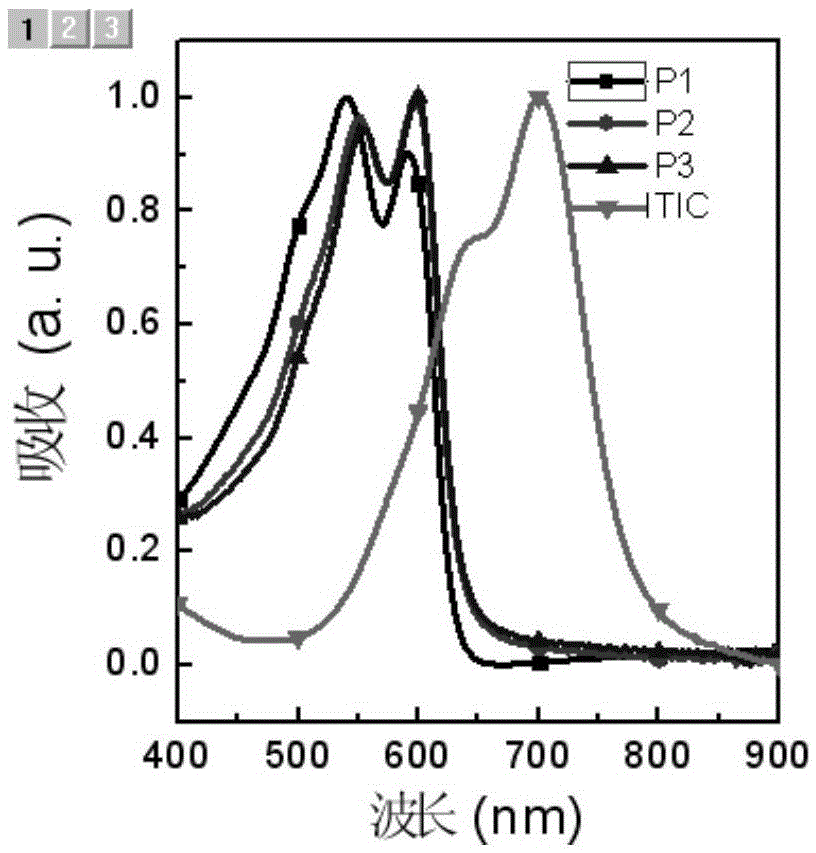
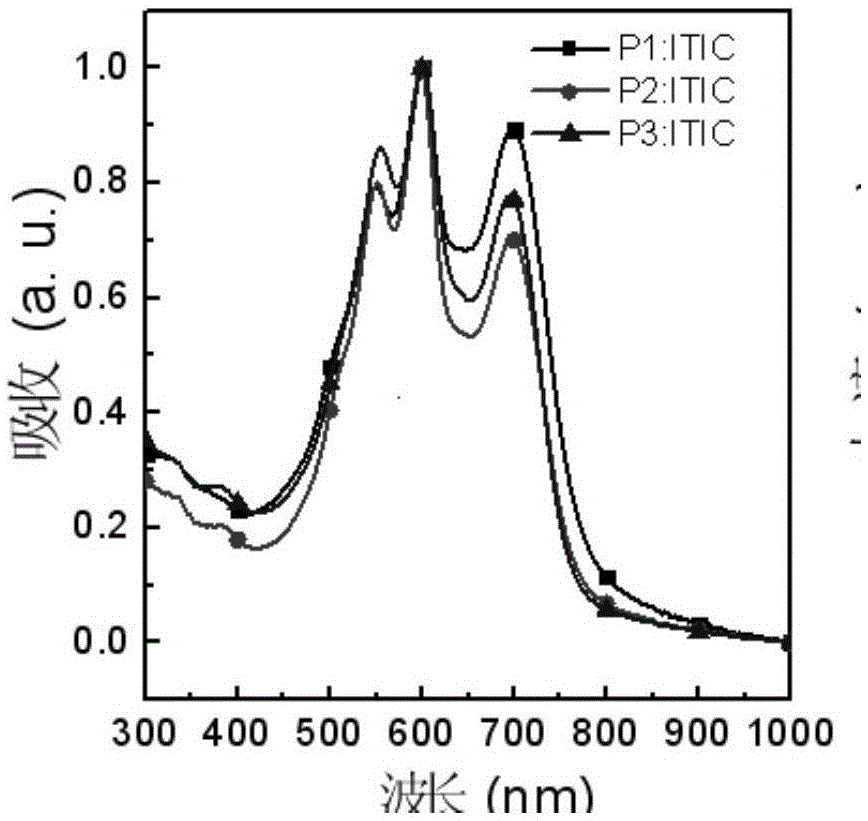
Benzotriazole-containing conjugated polymer and preparation method and application thereof in non-fullerene polymer solar cells
A technology of conjugated polymers and compounds, used in circuits, photovoltaic power generation, electrical components, etc., can solve the problems that devices cannot effectively utilize sunlight and are not efficient enough.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] The synthesis of polymer shown in embodiment 1, formula P1
[0102]
[0103] According to the above reaction equation, refer to the literature (Chem. Mater., 2012, 24 (16), 3247-3254), take 0.3mmol of each monomer M1 and M2, dissolve it in toluene (8mL) and DMF (2mL) After mixing the solvents, evacuate the air with argon for 5 minutes, then add the catalyst tetrakis(triphenylphosphine) palladium (0) (20 mg) and continue to evacuate the air for 25 minutes, then stop the polymerization after 14 hours at the reflux temperature of toluene. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Precipitate in methanol after dissolving with chloroform at last, filter, obtain the polymer shown in the formula P1 of red solid powder after vacuum drying one day, productive rate is 89%, GPC:Mn=57.5K; Mw / Mn=1.8. Anal. Calcd f...
Embodiment 2
[0104] The synthesis of polymer shown in embodiment 2, formula P2
[0105]
[0106] Carry out according to the above reaction equation, take 0.3mmol each of monomer M3 and M2, dissolve it in a mixed solvent of toluene (8mL) and DMF (2mL), exhaust the air with argon for 5 minutes, and then add catalyst tetrakis (triphenyl Phosphine)palladium(0) (20 mg) was then further evacuated for 25 minutes, and then the polymerization was stopped after 14 hours at the reflux temperature of toluene. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Precipitate in methanol after dissolving with chloroform at last, filter, obtain the polymer shown in the formula P1 of purple-red solid powder after vacuum drying one day, productive rate is 95%, GPC: Mn=23.9K; Mw / Mn=2.2 .Anal.CalcdforC 64 h 77 f 2 N 3 S 8 (%): C, 64.99; H, 6.56; ...
Embodiment 3
[0107] The synthesis of polymer shown in embodiment 3, formula P3
[0108]
[0109] According to the above reaction equation, take 0.3mmol each of monomer M4 and M2, dissolve it in a mixed solvent of toluene (8mL) and DMF (2mL), exhaust the air with argon for 5 minutes, and then add the catalyst tetrakis (triphenyl Phosphine)palladium(0) (20 mg) was followed by further evacuation of air for 25 minutes, and then the polymerization was stopped after 12 hours at the reflux temperature of toluene. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Precipitate in methanol after dissolving with chloroform at last, filter, obtain the polymer shown in the formula P3 of purple-red solid powder after one day of vacuum drying, productive rate is 98%, GPC: Mn=34.2K; Mw / Mn=2.2 .Anal.CalcdforC 77 h 93 f 2 N 3 S 8 (%): C, 66.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com