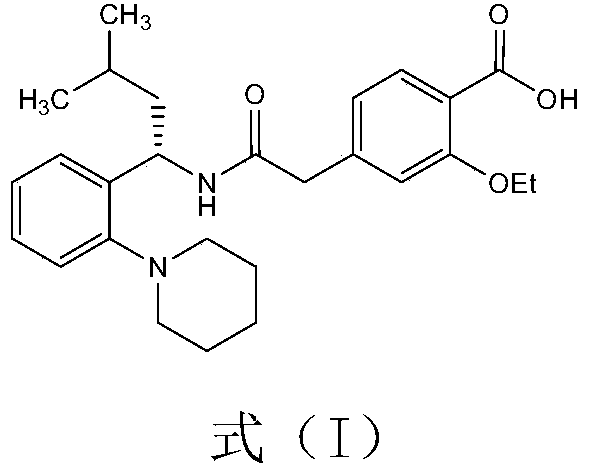
Stable repaglinide pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the field of solid preparations, can solve the problems of reduced uniformity of preparation content, different particle sizes, sticky materials, etc., and achieve the effects of improving mixing uniformity, controllable production process, and product quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
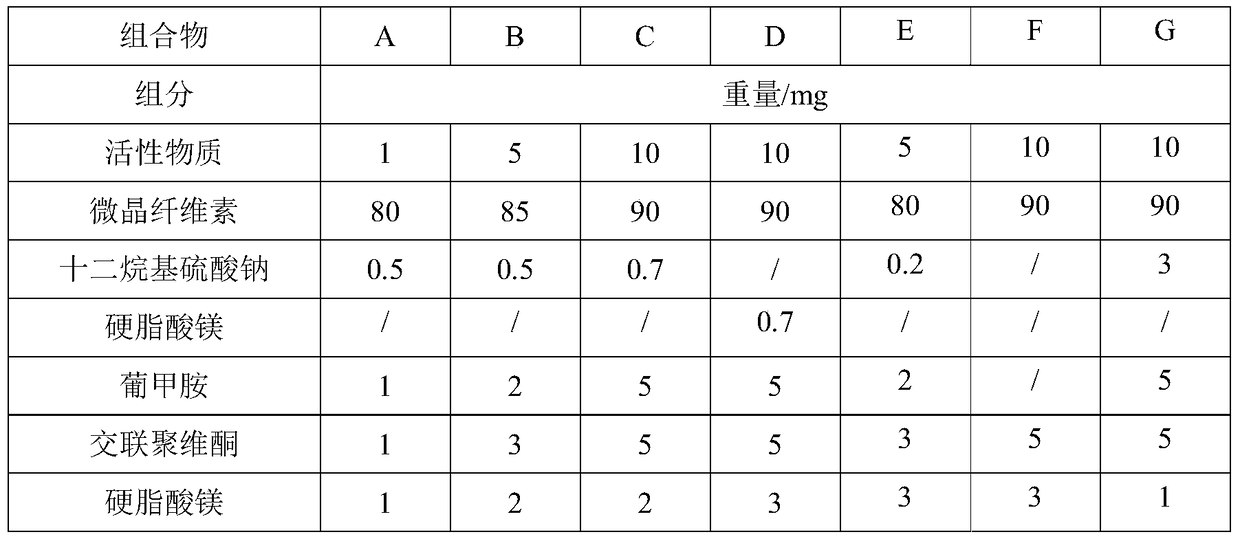
[0039] prescription:
[0040]
[0041] Take the material according to the above prescription, mix the active substance, sodium lauryl sulfate and meglumine evenly and pulverize it, then mix it with microcrystalline cellulose and carry out dry granulation, then add crospovidone and magnesium stearate Mix to obtain the final mixture.
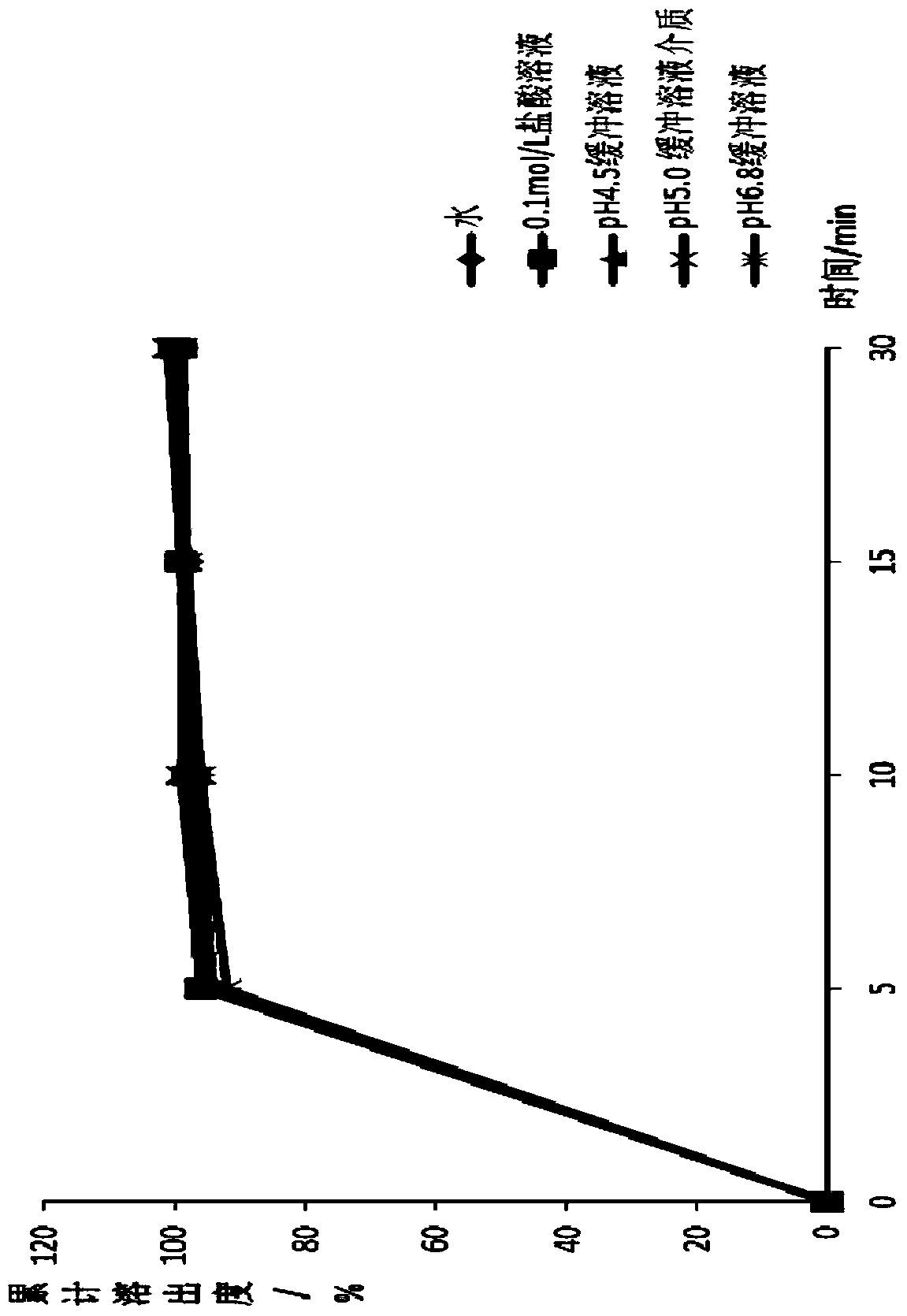
[0042] The A-D production process is smooth and the product quality is good. The particle results are shown in Table 1 below.
[0043] Table 1 Example 1 experiment related results
[0044]
[0045] Note: The particle content in this article refers to the measured value / theoretical value of the weight percentage of API in the composition in this field. The theoretical value is also called the labeled amount, and it is usually based on the proportion of API in the formulation design.
[0046] From the above data, it can be seen that sodium lauryl sulfate acts as a lubricant during the dry granulation process, so sodium lauryl sulfate in the...
Embodiment 2
2
[0054] Take the material according to the above prescription, mix the active substance, sodium lauryl sulfate and meglumine evenly and pulverize it, then mix it with microcrystalline cellulose and carry out dry granulation, then add crospovidone and magnesium stearate Mix to obtain the final mixture. Tablet compression was performed to determine the dissolution rate.
[0055] Meanwhile, above-mentioned prescription K is carried out as follows preparation:
[0056] Take the material according to the above K prescription, mix the active substance, sodium lauryl sulfate, meglumine, and crospovidone evenly and pulverize it, then mix it with microcrystalline cellulose and perform dry granulation, and then add stearic acid Magnesium is mixed to obtain the final mixture, which is the "K-1 prescription". Tablet compression was performed to determine the dissolution rate.
[0057] The above-mentioned obtained granules are compressed into tablets, and the results of the dis...
Embodiment 3
[0064] In order to more clearly illustrate the advantages of the prescription and process of this patent, the comparison of wet granulation and dry granulation prescription process is as follows:
[0065] prescription:
[0066] combination
M
N
components
Weight / mg
Weight / mg
active substance
10
10
90
91
0.7
0.7
meglumine
5
5
Crospovidone
5
5
3
3
[0067] Preparation Process:
[0068] 1. The above composition M was prepared into granules according to the process of Example 1, and pressed into tablets;
[0069] 2. The active substance, microcrystalline cellulose, sodium lauryl sulfate, meglumine, and crospovidone in the above composition N are subjected to pure water wet granulation, dried, and granulated with magnesium stearate mixed, compressed into tablets;
[0070] The above-mentioned prescriptio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com