Catechol derivative prepared using tyrosinase, method for preparing same, and application of same
A catechol-type, tyrosinase technology, which is applied in the directions of medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, can solve the difficulties of tyrosinase inactivation and accumulation of catechol-type structural substances, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Solubility change of initial substrate (monophenolic structure substance) according to pH
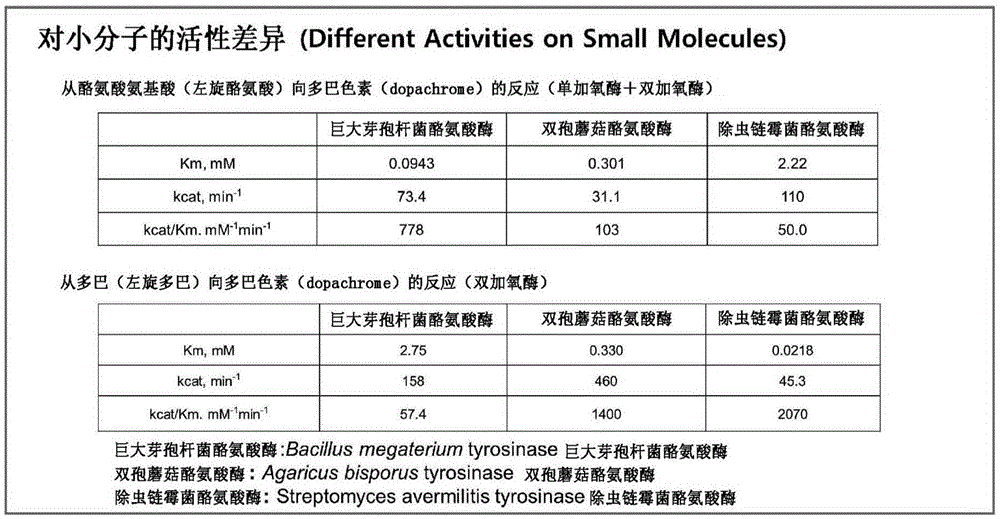
[0093] Daidzein (daidzein), which is an isoflavone series, has a low solubility in water, so in neutral water or a buffer solution at room temperature, it can be Dissolve about 300uM. However, it can be confirmed as follows: in the case of using a high-concentration boric acid buffer, the solubility increases as the pH becomes higher. In pH 9, the solubility increases more than ten times at a level of 5 mM, and in pH 10, the solubility increases at a level of 30 mM. Increased 100 times. Tyrosinase, unlike other monooxygenases, is active over a wide pH range, allowing the use of high concentrations of initial substrate to increase reaction productivity.
[0094] In pH 9, which is the range of tyrosinase activity, an in vitro (invitro) reaction of 5 mL containing 300 μM daidzein and 100 nM tyrosinase produced 15 mg of 3'-o-hydroxydaidzein per hour, whereas, The in vitro (invit...
Embodiment 2
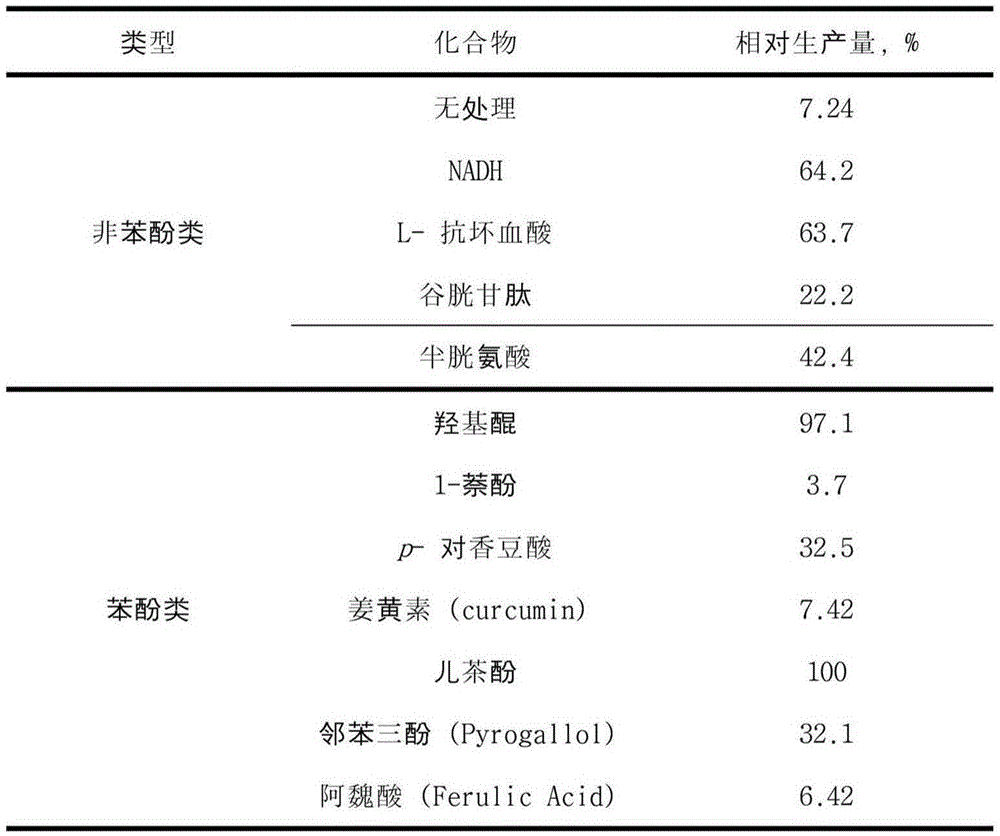
[0096] Comparative experiment on the yield of catechol-type structural substances according to the reducing agent
[0097] The cells expressing the tyrosinase derived from Streptomyces avermitilis cultured according to the expression method 1 clearly shown above were not disrupted, but only the cells were washed, and 0.1 mM resveratrol, 1 mM various reducing agents were added (Reduced nicotinamide adenine dinucleotide, L-ascorbic acid, glutathione, cysteine, hydroxyquinone, 1-naphthol, p-coumaric acid, curcumin, catechol, pyrogallol or ferulic acid) to produce picetanol. After the reaction, after using the same amount of ethyl acetate (EA, ethylacetate) or non-polar solvent to extract, use high-performance-liquid chromatography quantitative analysis to compare the production efficiency of piceatanol based on the addition of reducing agent, and make it explicit in figure 2 middle.
Embodiment 3
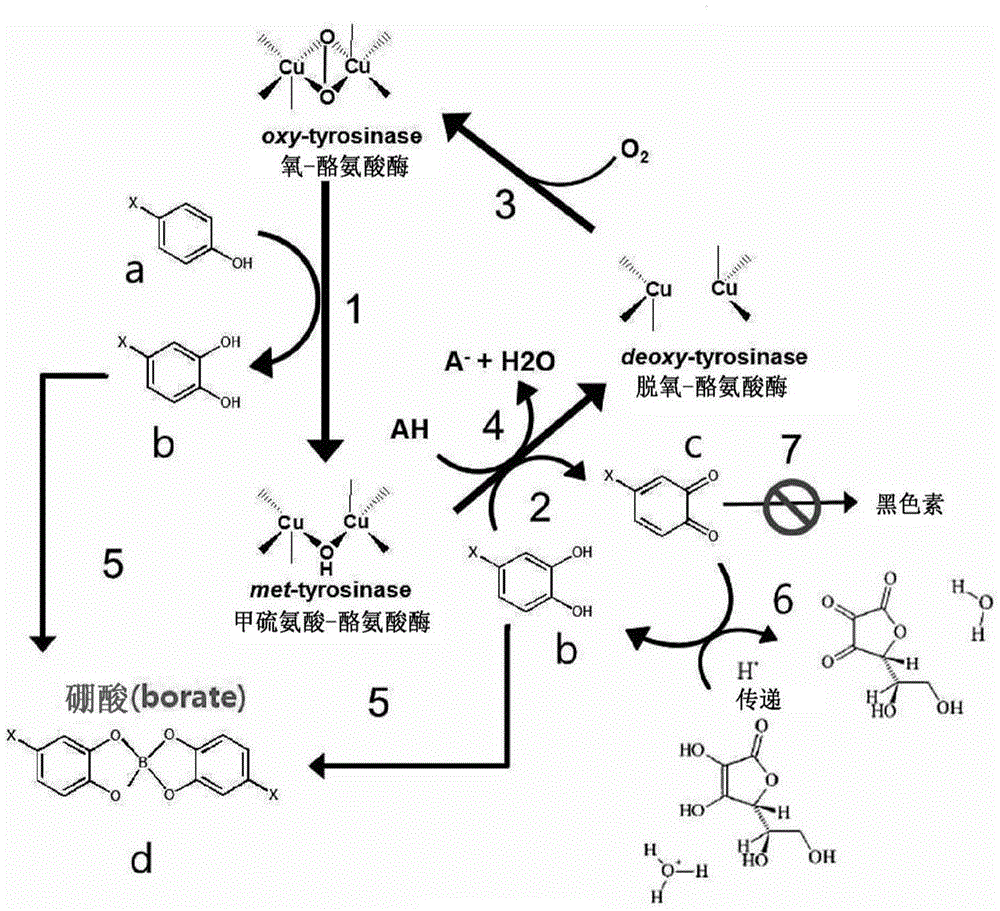
[0099] Coordination Bonds of Catechol-type Structural Substances
[0100] As in reaction method 1, 200nM of purified tyrosinase derived from bone marrow transplantation (BMT) was used to carry out hydroxylation reaction of 500uM daidzein, and at this time, boronic acid was added to induce coordination bonds. Such as Figure 5 As shown in part (a), it was confirmed by high-performance-liquid chromatography that 3'-o-hydroxydaidzein, which forms a coordinate bond in the alkaline reaction solution, has hydrophilicity, so it is not extracted into an organic solvent. and, in Figure 5 In part (b), it was confirmed that when 2M HCl was treated, the coordination bond was released, and 200uM 3'-o-hydroxydaidzein was detected.
[0101] And, utilize the bone marrow transplantation tyrosinase of purification 300nM, also make 1mM genistein and apigenin carry out hydroxylation reaction, as Figure 6 As shown, it is confirmed by high performance-liquid chromatography that hydroxylated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com