Preparation method of blended cross-linked anion exchange membrane
An anion exchange membrane, toluene technology, used in electrical components, circuits, battery electrodes, etc., can solve the problems of poor chemical stability and affect the life of fuel cells, and achieve the effects of low swelling rate, excellent chemical stability, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
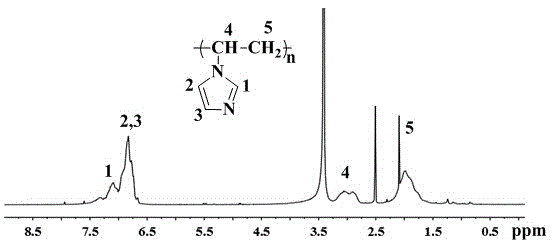
[0025] Add 0.2g of azobisisobutyronitrile initiator and 30ml of toluene into a dry and clean 100ml three-neck flask respectively, and stir magnetically. After the initiator is completely dissolved, add 20ml (20.78g) of vinylimidazole under the protection of nitrogen. The reaction system was heated up to 70°C, and reacted at this temperature for 20 hours. After cooling to room temperature, it was slowly poured into acetone, stirred, and filtered with suction. The filter cake was washed repeatedly with acetone, placed in a vacuum oven, and dried at 60°C. , to obtain 18.9 g of polyvinylimidazole product.
[0026] figure 2 is the proton nuclear magnetic resonance spectrum of the prepared polyvinylimidazole, the attribution of each peak has been marked in the figure, and the analysis result is consistent with the structure of polyvinylimidazole.
Embodiment 2
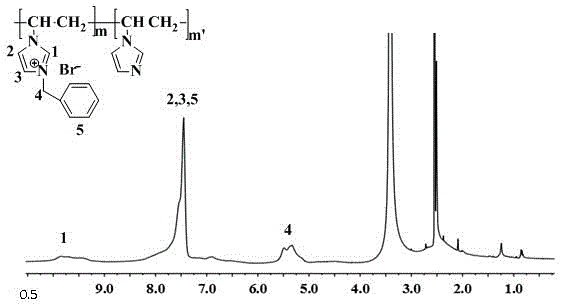
[0028] In a dry and clean 100ml three-necked flask, add 3.76g (40mmol) of the polyvinylimidazole prepared in Example 1, add 35ml of dimethyl sulfoxide under nitrogen protection, magnetically stir, and heat to completely dissolve the polyvinylimidazole After cooling to room temperature, 5.13 g (30 mmol) of benzyl bromide was added to the reaction flask, and the reaction was carried out at 80° C. for 10 hours. Cool to room temperature, slowly pour the resulting solution into acetone, filter with suction, wash the filter cake fully with acetone to remove unreacted benzyl bromide and dimethyl sulfoxide, put it in a vacuum oven, and dry it at 60°C Dry to obtain polyvinylimidazolium bromide.
[0029] image 3 It is the proton nuclear magnetic resonance spectrum of the brominated polyvinylimidazolium salt. The attribution of each peak has been marked in the figure. According to the area ratio of the two peaks of H4 and H1, the quaternization rate can be calculated as 68%. .
Embodiment 3
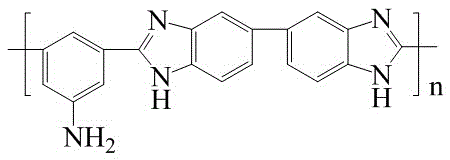
[0031] (1) Add 0.1g polybenzimidazole containing side amino groups respectively in a dry and clean Erlenmeyer flask (the chemical structure of polybenzimidazole containing side amino groups is as follows: figure 1 Shown) and 2ml dimethyl sulfoxide, heat and stir to make it fully dissolved, add 0.9g brominated polyvinylimidazolium salt and 18ml Dimethyl sulfoxide, heated and stirred to fully dissolve. After cooling to room temperature, mix the two solutions with each other, and add 0.1235g of 1,6-dibromohexane and 0.01g of bisphenol A epoxy resin to the mixture, stir for 30min with a magnet, filter, and then cast On a dry and clean glass plate, place it in a blast oven, dry it at 80°C for 10 hours, then transfer it to a vacuum oven, and dry it at 110°C for 3 hours to obtain polyvinyl benzyl imidazolium bromide / Blended cross-linked anion exchange membrane with polybenzimidazole mass ratio of 9:1.
[0032] (2) At room temperature, soak the blended cross-linked anion exchange m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com