In situ gel for injection containing polymyocytes and preparation method thereof
A technology of in situ gel and polyinocytes, which is applied in the direction of medical preparations containing active ingredients, medical preparations with inactive ingredients, pharmaceutical formulas, etc., can solve the limitations of the development and Application, easy precipitation, solubility drop, etc., to achieve the effect of improving animal compliance, simple preparation process, and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
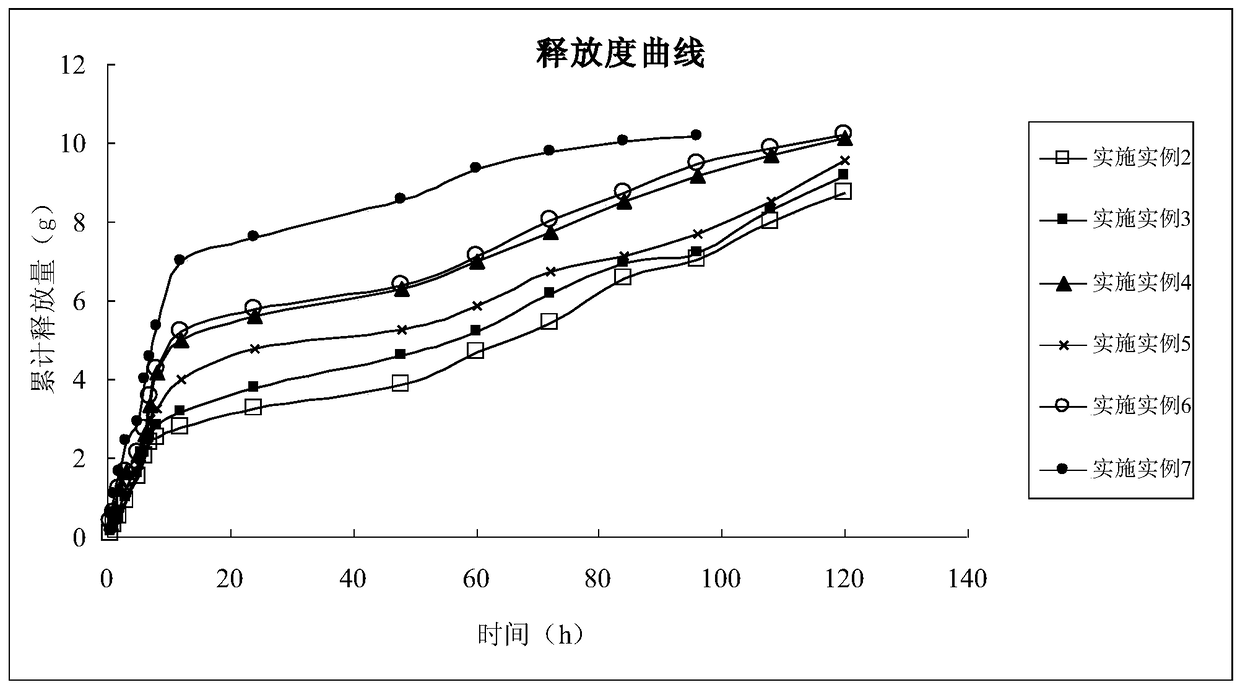
Image
Examples
Embodiment 1
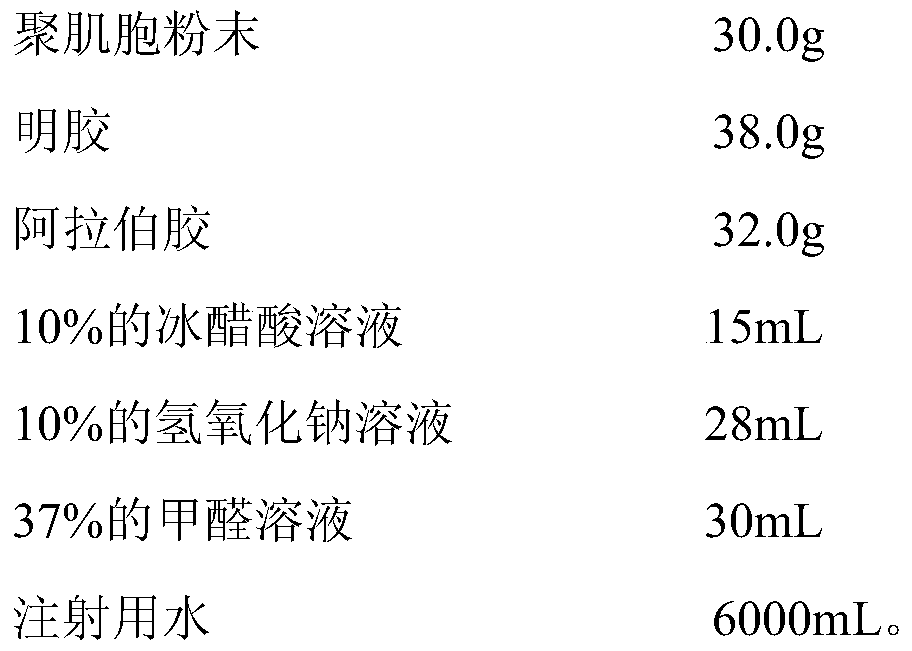
[0028] Polymyocyte Microcapsules
[0029] prescription:
[0030]
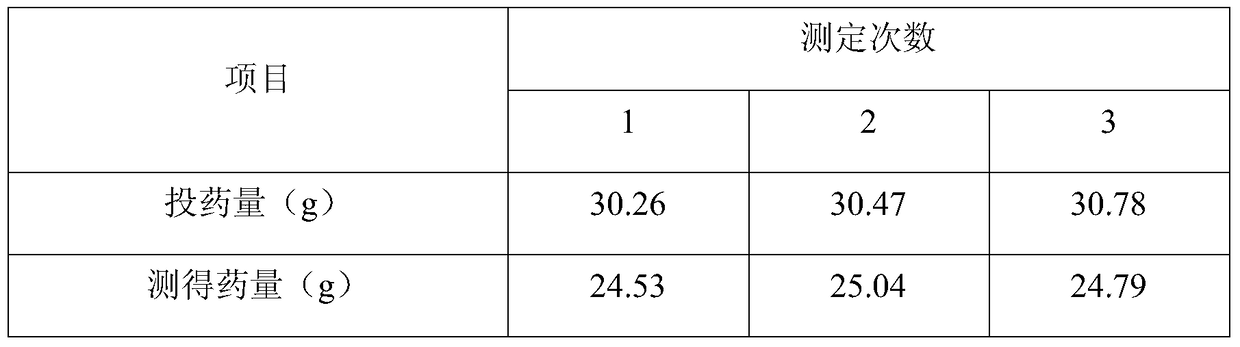
[0031] Preparation method: (a), take the prescribed amount of gelatin and gum arabic and dissolve them in 800ml of water for injection respectively to obtain gelatin aqueous solution and gum arabic aqueous solution, mix the two solutions at a volume ratio of 1:1, keep the temperature at 55°C, Continue to stir; (b), dissolving the polymyocytes of the prescription amount in the gelatin-gum arabic mixed solution under stirring, continue to stir for 30 minutes; (c), add 10% glacial acetic acid solution of the prescription amount, adjust the solution The pH of the solution is between 3-4, take a sample to observe the formation of capsules, add 4400ml of water for injection after formation of capsules, stir evenly, place in an ice bath and quickly cool to below 10°C, add 30mL of formaldehyde solution, and stir for 30 minutes; (d ), adding 10% sodium hydroxide solution to adjust the pH of the solution at 8-9, soli...
Embodiment 2
[0037] In situ gel for polysarcoma injection (each 1mL contains 5 mg of polysarcoma)
[0038] The prescription is as follows:
[0039]
[0040] Preparation method: (a), take prescription amount of disodium hydrogen phosphate, sodium dihydrogen phosphate, dissolve in 700mL water for injection to prepare a buffer solution with pH6.8, dissolve the prescription amount of potassium sorbate in the buffer solution, and then add Stir the prescribed amount of polyinosin powder evenly to obtain solution A; (b), sprinkle the prescribed amount of gel matrix P407, P188 and hydroxypropyl methylcellulose on solution A, and refrigerate at about 4°C until obtained Clarify, no agglomerate, uniformly dispersed solution, filter to obtain solution B; (c), add the polyinocytic microcapsules of the prescribed amount into solution B, stir until the dispersion is uniform, add the remaining amount of water for injection, mix Homogenize and sterilize to obtain 1000mL in situ gel for injection contai...
Embodiment 3
[0042] In situ gel for polysarcoma injection (each 1mL contains 6 mg of polysarcoma)
[0043] The prescription is as follows:
[0044]
[0045]
[0046] Preparation method: (a) Dissolve disodium hydrogen phosphate and sodium dihydrogen phosphate in the prescribed amount, dissolve them in 700mL water for injection to prepare a pH6.9 buffer, dissolve the prescribed amount of potassium sorbate in the buffer, and add the prescribed amount Amount of polymyocyte powder, stirred evenly, to obtain solution A; (b) Sprinkle prescription amount of gel matrix P407, P188 and povidone on solution A, and refrigerate at about 4°C until a clear, lump-free, The uniformly dispersed solution is filtered to obtain solution B; (c) add the prescribed amount of polysarcoma microcapsules to solution B, stir until uniformly dispersed, add the remaining amount of water for injection, mix well, and sterilize to obtain 1000mL in situ gel for injection containing polymyocytes; finally dispensed acco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com