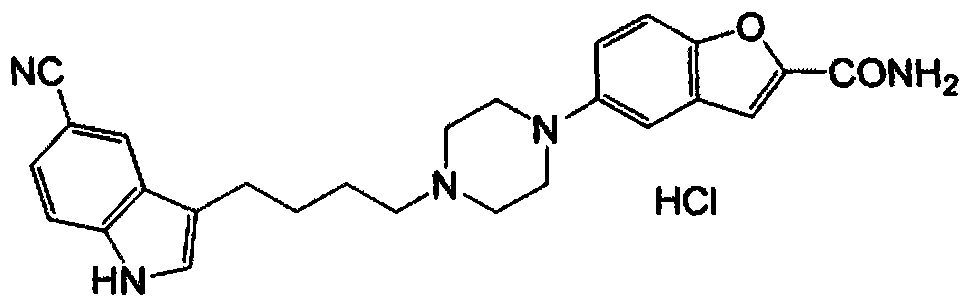
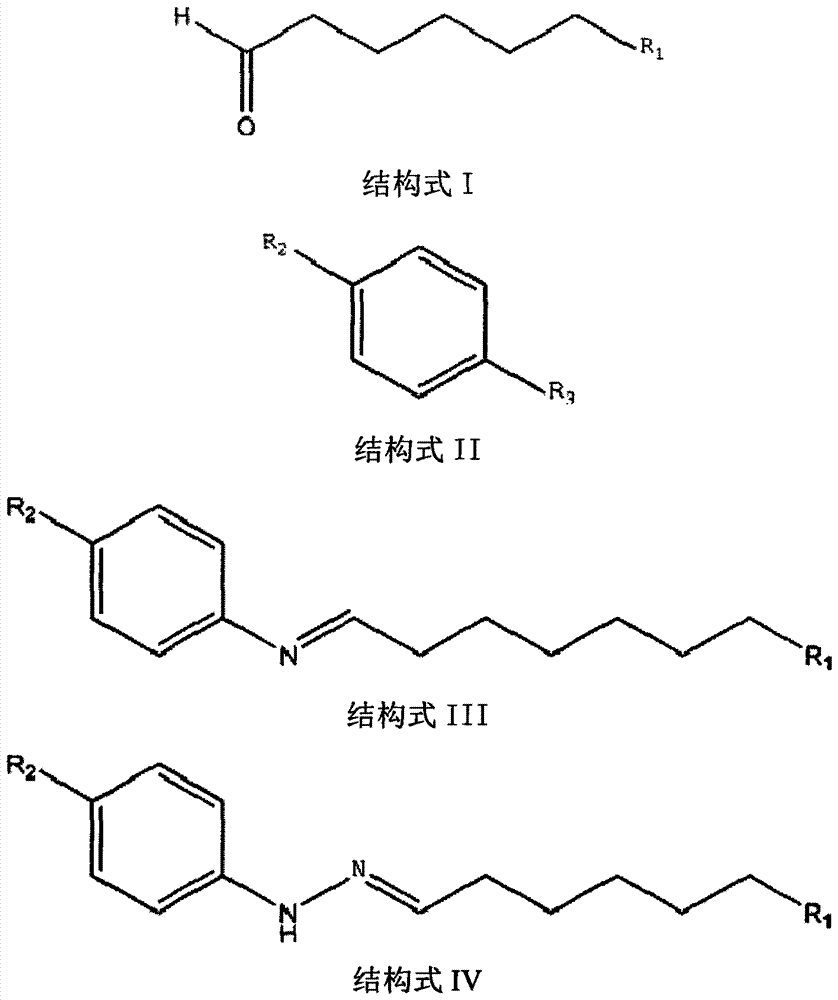
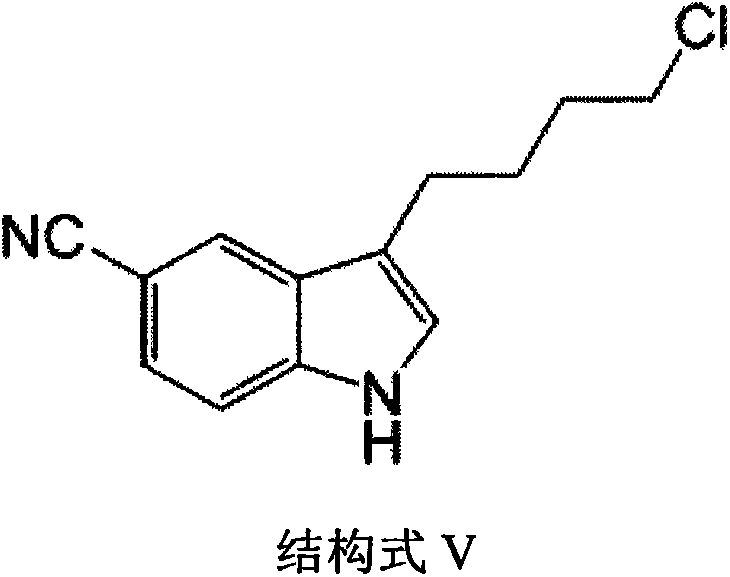
A kind of preparation method of vilazodone intermediate
A technology of vilazodone and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of unsuitability for industrial production, difficult operation of red aluminum, low yield and the like, and achieves short synthesis route, low cost and high yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of 6-chlorohexanal-4-cyanophenylhydrazone
[0029] Take 21.40g (0.126mol) of 4-cyanophenylhydrazine hydrochloride and add it to a 250mL three-necked bottle, 2 Under protection, add 140mL methanol, 13.40g Et 3 N (0.133mol) was stirred into a solution, cooled in an ice bath, and 6-chlorohexanal (23.30g, 0.174mol) was added dropwise at 0-5°C, after the addition was complete, stirred at this temperature for about 15 minutes, TLC showed a reaction Complete (petroleum ether: ethyl acetate = 1: 1), stop the reaction. The reaction liquid was poured into ice water, kept stirring at -10-0°C, and a solid was precipitated, filtered, and vacuum-dried to obtain 26.6 g of hydrazone, with a yield of 84%. The obtained hydrazone is a mixture of Shuangjian Shun and trans isomers, the ratio of the two is 1:4, and the identification data of the cis structure is as follows:
[0030] 1 H-NMR, (400MHz CDCl 3 ppm) 7.48 (d, J = 8.6Hz 2H), 7.13 (t, J = 5.1Hz, 1H), 6.99 (d, J = 8.6H...
Embodiment 2
[0034] Synthesis of 3-(4-chlorobutyl)-5-cyanindole
[0035] Put 10.00 g of hydrazone (40 mmol) in a 500 mL flask, add 150 mL of acetonitrile and 14.80 g (44 mmol) of polyphosphoric acid, and heat until the acetonitrile refluxes. After reacting for about one hour, HPLC showed that the reaction was complete (acetonitrile:water=80:20). Cool the reaction solution to room temperature, distill off acetonitrile under reduced pressure, add water (200 mL) and ethyl acetate (200 mL) to dissolve the residue, separate the water layer, extract the water layer with ethyl acetate (3×150 mL), combine the organic layers, and use Wash with water (2×200 mL), wash with saturated sodium chloride solution (200 mL), dry the organic layer over anhydrous sodium sulfate, filter, and concentrate the filtrate to obtain a yellow solid. Add 1:1 (V / W) methanol to the product, heat to reflux to dissolve, cool to room temperature to precipitate a solid, filter and dry in vacuo to obtain 5.59 g of indole comp...
Embodiment 3
[0038] Synthesis of Vilazodone
[0039] Add 3-(4-chlorobutyl)-5-cyanindole 36.10g (155mmol), 5-(piperazin-1-yl)benzofuran-2-carboxamide 36.00g (147mmol ), 25.50g of sodium acetate (310mmol), dissolved in 500mL of DMAC under stirring, reacted at 90°C (external temperature) for 18 hours, cooled the reaction solution to room temperature, added 1L of water, extracted with ethyl acetate (3 × 400mL), combined organic The organic layer was washed with water (2×300 mL), washed with saturated sodium chloride solution (200 mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain a yellow solid. Using a mixed solvent for purification, first dissolve the solid in THF, add acetone and methanol under stirring, stir to precipitate the solid, filter, vacuum dry and weigh to obtain vilazodone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com