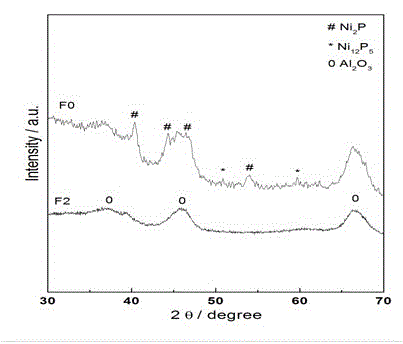
Non-noble metal selective hydrogenation catalyst, preparation method and application thereof
A technology for hydrogenation catalysts and non-noble metals, applied in chemical instruments and methods, physical/chemical process catalysts, hydrocarbons, etc., can solve the problems of high catalyst cost, limited application, expensive precious metals, etc., and achieve low catalyst cost, The effect of low hydrogenation loss rate and low isomerization rate of butene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
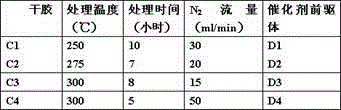
[0028] Catalyst preparation steps (1)
[0029] Weigh nickel nitrate hexahydrate and add 500ml deionized water according to the weight shown in Table 1, then add 0.05mol / L nitric acid to adjust the pH value to 2-3, then add diammonium hydrogen phosphate and nitric acid hexahydrate according to the weight shown in Table 1 Cerium, citric acid monohydrate, and then stirred at room temperature for 1 hour to obtain solutions A1-A4, respectively.
[0030] surface Catalyst Preparation Table
[0031]
[0032] Add 77g, 72g, 67g and 78g of aluminum hydroxide powder to solutions A1-A4 to disperse them in the solution to obtain slurries B1-B4 respectively, then transfer the obtained slurries into a rotary evaporator and evaporate the water to dryness at 85°C , and then dried at 120°C for 24 hours to obtain dry glue C1-C4.
[0033] Catalyst Preparation Step (2)
[0034] Place the dry glue C1-C4 in a tubular heating furnace under continuous flow of N 2 In the atmosphere, treat...
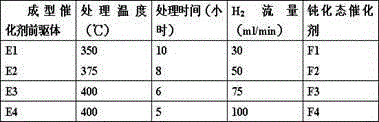
Embodiment 5-10
[0048] The reactivity of the catalyst was evaluated by selective hydrogenation of butadiene. Before each catalyst evaluation, the catalyst needs to be pretreated in a hydrogen atmosphere at 200°C for 2 hours, and then cooled to the reaction temperature. A mixed carbon four is used as a raw material, and the composition of the raw material is shown in Table 4. The reaction products were analyzed online by Agilent7890 gas chromatograph.
[0049] Table 4 raw material composition
[0050]
propane Isobutane n-butane trans-2-butene 1-butene Isobutylene cis-2-butene Butadiene Composition / wt% 0.06 34.23 11.55 11.99 11.93 15.74 14.15 0.35
[0051] Using F1-F4 as the catalyst, F0 as the reference, and the above-mentioned mixed carbon four as the raw material, the reaction temperature is 60-100°C, the total pressure is 1.5-2.0MPa, and the space velocity is 5-10h -1 , Under the condition that the hydrogen / butadiene molar ratio is 1.0-2.0, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com