Aromatic diamine monomer and preparation method thereof
A technology of aromatic diamine and monomer, which is applied in the field of diamine monomer and its preparation, can solve the problems that hinder the pace of research and development of aramid fiber, the damage of fiber performance, broken filaments and wool, etc., and achieve improvement Overall performance, simplification of the dissolution process, and the effect of particles that are not easily gelled
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
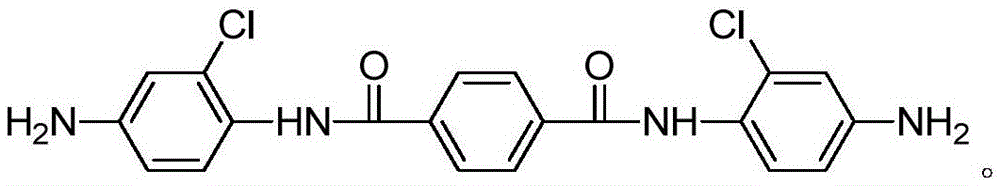
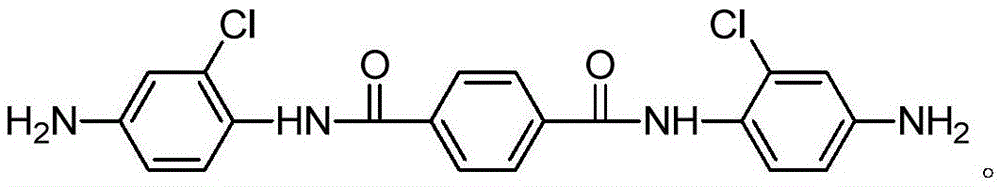
[0024] Add 2-chloro-4-nitroaniline (51.9g, 0.3mol), pyridine (23.7g, 0.3mol) and tetrahydrofuran (215ml) in the three-necked round-bottomed flask, then use the dropping funnel to slowly drop the solution dissolved in tetrahydrofuran ( Terephthaloyl chloride (20.3g, 0.1mol) in 108ml), the rate of addition was 120ml / h, and heated at 25°C for 12h. After the reaction, the solid was filtered and washed three times with water and acetone. Drying under low temperature gave a yellow solid which was the nitro compound (43 g, 90.5%). Under the protection of argon (three deoxygenation), add ethanol (280ml) palladium carbon (0.53g) to the nitro compound (23.8g, 0.05mol), react at 80°C for 8 hours under hydrogen, and filter after the reaction The crude product was obtained, and then separated by column chromatography (the eluent was dichloromethane / ethyl acetate with a volume ratio of 2:1) to obtain a light green solid that was N,N'-bis(4-amino-2-chloro phenyl) terephthalamide.
Embodiment 2
[0026] Add 2-chloro-4-nitroaniline (60.6g, 0.35mol), pyridine (23.7g, 0.3mol) (26.1g, 0.33mol) and tetrahydrofuran (215ml) in the three-necked round bottom flask, then use the dropping funnel Slowly add terephthaloyl chloride (20.3g, 0.1mol) dissolved in tetrahydrofuran (108ml) dropwise at a rate of 120ml / h, heat at 25°C for 12h, and filter to obtain a solid after the reaction, then water and Washed three times with acetone and dried under vacuum to obtain a yellow solid which is the nitro compound (43 g, 90.5%). Under the protection of argon (three deoxygenation), add ethanol (280ml) palladium carbon (0.53g) to the nitro compound (23.8g, 0.05mol), react at 100°C for 8 hours under hydrogen, and filter after the reaction The crude product was obtained, and then separated by column chromatography (the eluent was dichloromethane / ethyl acetate with a volume ratio of 2:1) to obtain a light green solid that was N,N'-bis(4-amino-2-chloro phenyl) terephthalamide.
Embodiment 3
[0028] Add 2-chloro-4-nitroaniline (69.2g, 0.4mol), pyridine (23.7g, 0.3mol) and tetrahydrofuran (215ml) in the three-neck round-bottomed flask, then use the dropping funnel to slowly drop the solution in tetrahydrofuran ( Terephthaloyl chloride (20.3g, 0.1mol) in 108ml), the rate of addition is 120ml / h, heated at 25°C for 12h, filtered to obtain a solid after the reaction, and then washed three times with water and acetone, and the Drying under low temperature gave a yellow solid which was the nitro compound (43 g, 90.5%). Under argon protection (three deoxygenation), add ethanol (280) palladium carbon (0.53g) in the nitro compound (23.8g, 0.05mol), under hydrogen condition, react at 100 ℃ for 8 hours, and the reaction ends After filtration, the crude product was obtained, and then separated by column chromatography (the eluent was dichloromethane / ethyl acetate with a volume ratio of 2:1) to obtain a light green solid that was N,N'-bis(4-amino-2 -chlorophenyl) terephthalamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com