Method for preparing univalent selective cation exchange membrane by electrodeposition
A cation exchange membrane and electrodeposition technology, applied in the field of ion exchange membranes, can solve the problems of short service life, high price, unfriendly environment, etc., and achieve excellent performance, prolong life, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
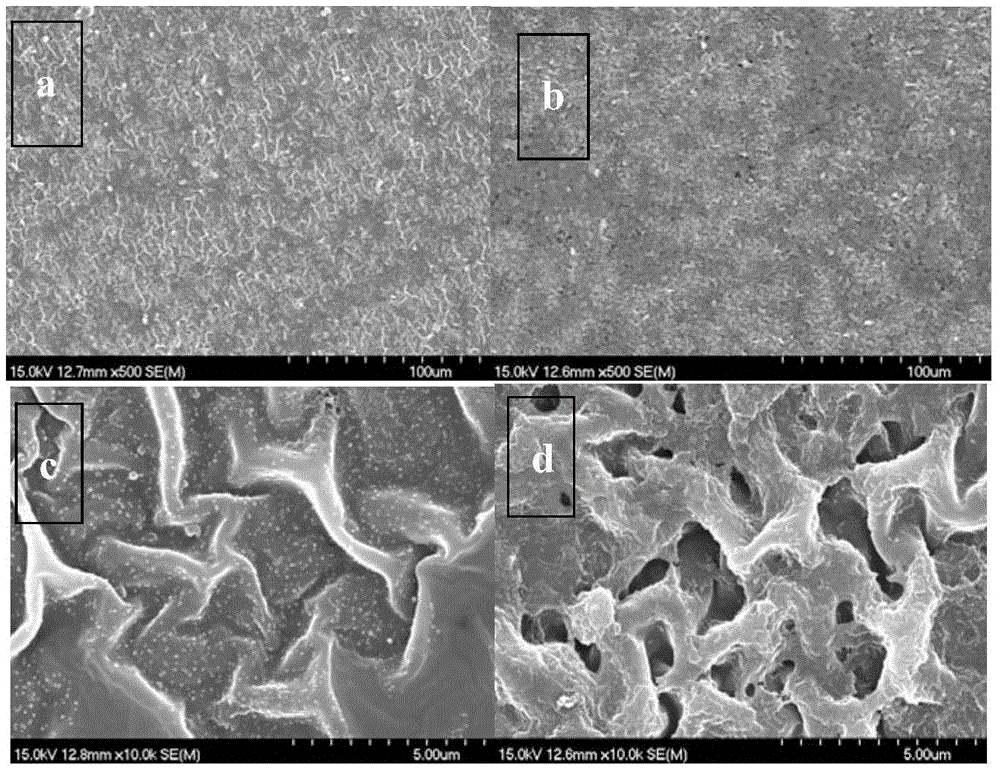
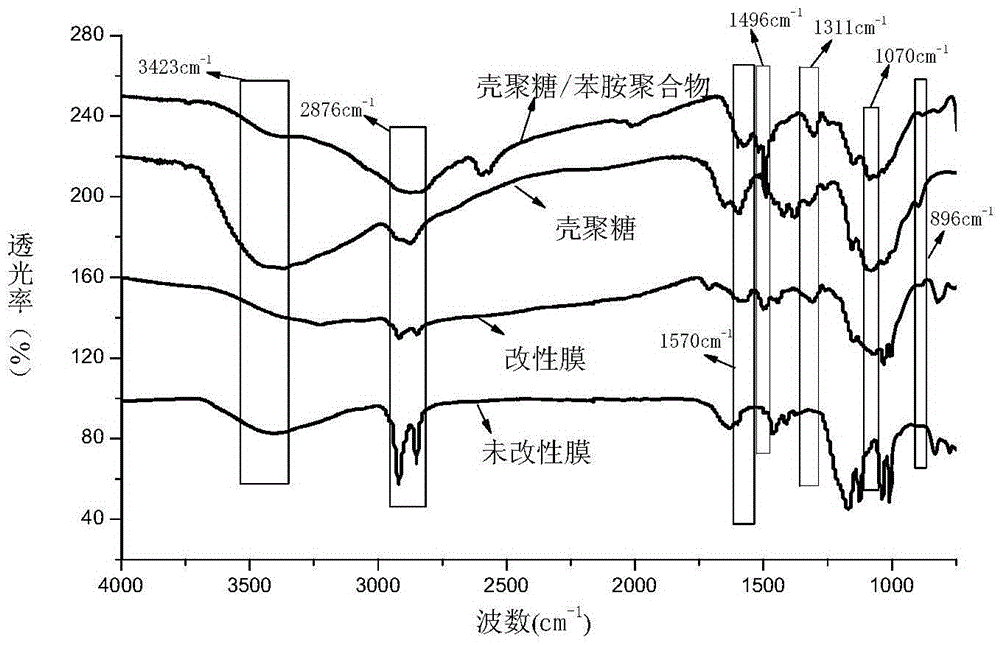
Image
Examples
Embodiment 1
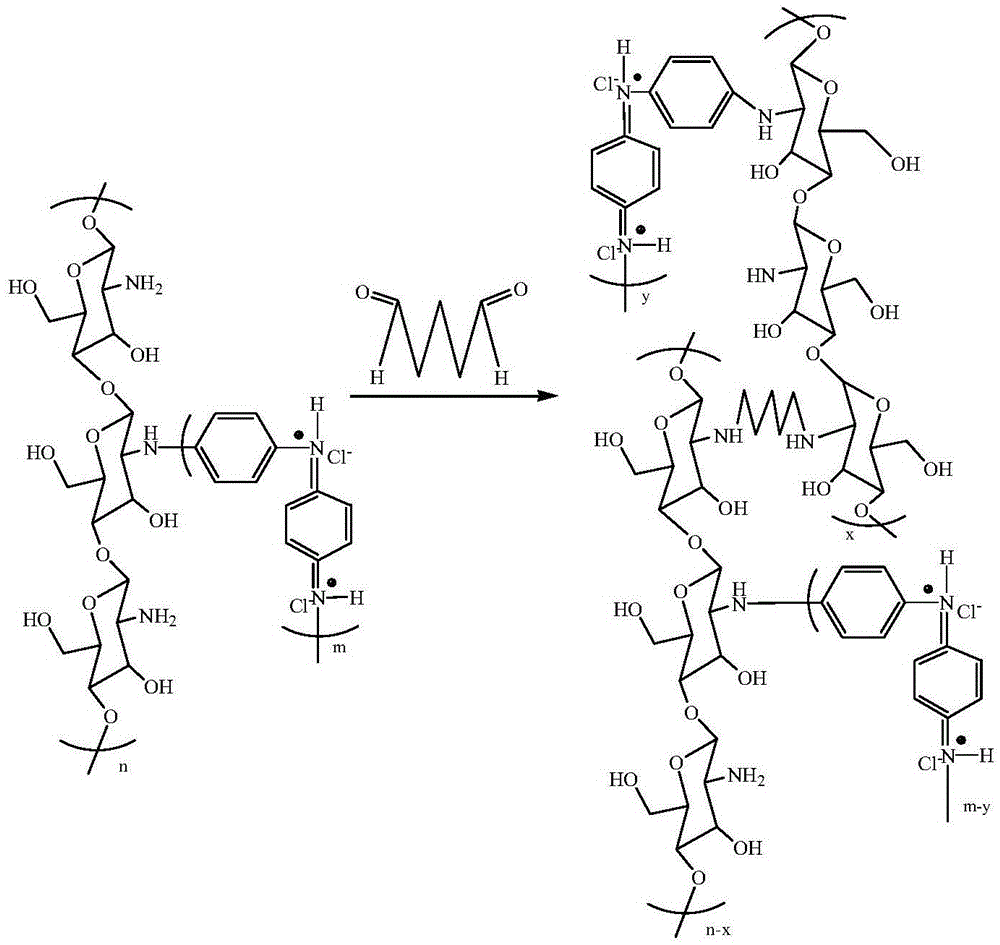
[0036] 1g chitosan is dissolved in the acetic acid solution of mass fraction 2% of 200mL to obtain chitosan solution, 0.578g aniline is dissolved in the 1M HCl solution of 200mL to obtain aniline solution, the above-mentioned 200mL aniline solution and the chitosan solution of 200mL After thorough mixing, at room temperature N 2Under protective conditions, slowly drop 100mL of 20g / L ammonium persulfate solution. After dropping, stir at room temperature for reaction, the reaction time is 10 h, after the reaction, the polymer solution is separated by centrifugation. The centrifugation speed is controlled at 10000rpm, and the centrifugation time is 10min. The separated polymer solid was redissolved in 2% acetic acid aqueous solution by mass fraction, and centrifuged again, so that unreacted substances were removed three times. The final polymer solid product obtained was dried in a drying oven at 50° C. for 24 hours for use.
[0037] The commercial homogeneous cation exchange ...
Embodiment 2
[0041] Five kinds of modified membranes prepared in Example 1 with different electrodeposition times and unmodified commercial homogeneous cation exchange membranes were respectively placed in a four-compartment electrodialysis device, and the effective area of the membrane was 5cm×5cm. Both sides are polar liquid chambers, add 0.5mol / LNa 2 SO 4 , the middle two compartments use 0.5mol / L NaCl as the measuring solution to measure the membrane resistance. A multimeter is connected to the Ag / AgCl electrode to measure the potential difference across the membrane. In the experiment, the constant current method was used to measure the voltage on both sides under the conditions of membrane and membrane, and the membrane resistance was obtained by using the voltage difference. The film resistance of different electrodeposition time such as Figure 5 shown.
[0042] Figure 5 It shows that as the electrodeposition time increases, the resistance of the film surface increases, whi...
Embodiment 3
[0044] The modified membrane prepared in Example 1 with an electrodeposition time of 4h is placed in a four-compartment electrodialysis device, such as Figure 4 as shown, Figure 4 Among them, M-CEM represents the modified membrane, AEM represents the anion exchange membrane, and the effective area of the membrane is 5cm×5cm. In the device, the dilute chamber is 200mL0.5MH 2 SO 4 , the concentrated chamber is 200mL containing 15g / LZnSO 4 0.5MH 2 SO 4 solution. Electrolyte is 0.5MK 2 SO 4 . The electrodialysis time is controlled to 100min, and the current density is 50mA / cm 2 .
[0045] As shown by the experimental results, the Zn of the film 2+ The leakage rate decreased from 21% of the original film before modification to 12.5%. Membrane resistance from 6.7Ωcm before modification 2 Rise to 9.6Ωcm 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com