Magnetic covalent organic framework nanocomposite material and preparation method and application
A technology of covalent organic framework and nanocomposite materials, which is applied in the fields of peptide preparation methods, organic chemistry, chemical instruments and methods, etc., can solve problems such as unstable structures and unsatisfactory enrichment of hydrophobic peptides, and achieve Rapid enrichment and separation, high yield and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of magnetic covalent organic framework nanocomposite, it comprises the steps:
[0035] (1) 8.1gFeCl 3 ·6H 2 O and 12.0g dispersant sodium acetate are added to 200mL ethylene glycol solution and mixed;
[0036] (2) Add 1.5g trisodium citrate dihydrate as electrostatic stabilizer;
[0037] (3) Then move the solution into an airtight heating container, and carry out a solvothermal reaction at 200°C for 8 hours to obtain ferric oxide nanoparticles;
[0038] (4) Disperse 50 mg of ferric oxide nanoparticles synthesized in 15 mL of isopropanol,
[0039] (5) Add 26.5 mg of 1,3,5-tris(4-aminophenyl)benzene and 15 mg of terephthalaldehyde, and disperse evenly by ultrasonication for 25 minutes at room temperature;
[0040] (6) Continue to sonicate, add 0.5mL acetic acid while sonicating, and then continue to sonicate for 20min.
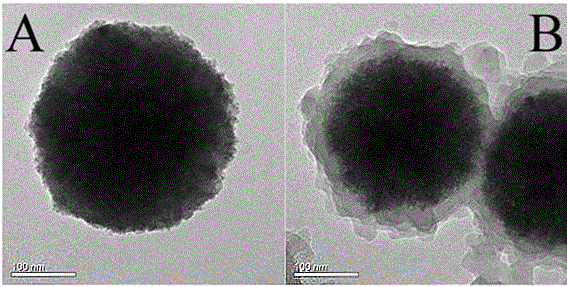
[0041] figure 1 A is a transmission electron microscope image of the iron ferric oxide nanoparticles synthesized in Example 1. It can...
Embodiment 2
[0045] Magnetic covalent organic framework nanocomposite for the enrichment of peptides containing benzene ring structure
[0046] A series of 5 mg magnetic covalent organic framework nanocomposites synthesized in Example 1 were weighed into 5 mL centrifuge tubes, and mixed with 1 mL of different concentrations of peptide L2 (FGFG) and peptide L7 (RPPGFSPFR) respectively. The mixture was placed on a shaker at room temperature for 2 h to ensure adsorption equilibrium. After adsorption equilibrium, a magnet was used to separate the mixture of magnetic particles and peptides, and the absorbance of the supernatant after the reaction was tested by a UV-visible spectrophotometer to calculate the adsorption amount. Such as Figure 4 As shown, the saturated adsorption capacity of magnetic covalent organic framework nanocomposites to peptide L7 is greater than 7.5 mg / g, while the saturated adsorption capacity of peptide L2 is only 1.5 mg / g, indicating that magnetic covalent organic fr...
Embodiment 3
[0048] Magnetic covalent organic framework nanocomposites for separation and enrichment of peptides containing benzene ring structure and actual serum samples
[0049] A series of 5 mg magnetic covalent organic framework nanocomposites synthesized in Example 1 were weighed into a 5 mL centrifuge tube and mixed with 1 mL standard solution (containing 50-fold diluted healthy human serum and 50 ppm of L7 (RPPGFSPFR)). The mixture was kept on a shaker at room temperature for 2 h to ensure adsorption equilibrium. After adsorption equilibrium, a magnet was used to separate the mixture of magnetic particles and peptides, and the supernatant was collected. The adsorbed material was eluted with 200ul eluent (50% acetonitrile + 50% water), mixed evenly, kept on a shaker for 1 hour, and the eluate was collected. Chromatographic separation of standards, supernatants and eluents was performed by high performance liquid chromatography. Figure 5 It is the chromatogram of standard solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com