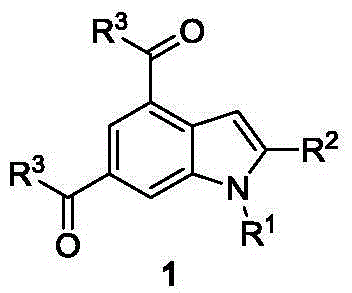
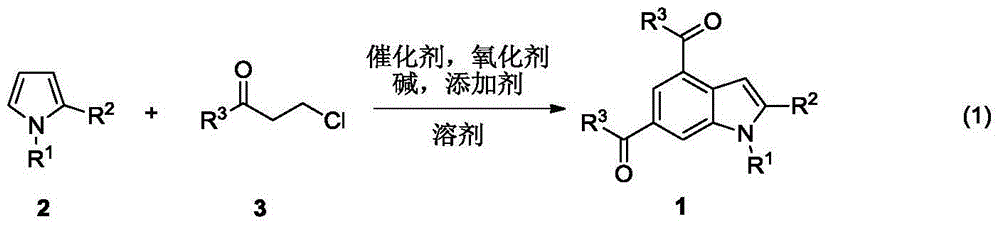
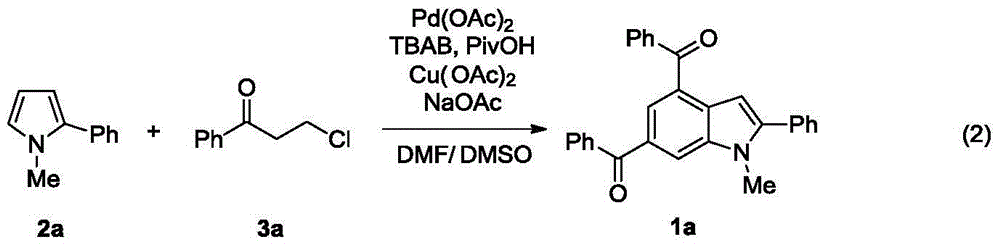
Synthesis method of indole derivative
A technology of indole derivatives and synthetic methods, applied in the direction of organic chemistry, can solve problems such as application limitations, and achieve the effects of simple operation, high product yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] In a 10mL Schlenk reaction flask, add palladium acetate Pd(OAc) successively under air 2 (4.5mg, 0.02mmol), N-methyl-2-phenylpyrrole 2a (0.2mmol), chloropropiophenone 3a (133mg, 0.8mmol), copper acetate Cu(OAc) 2 (145mg, 0.8mmol) sodium acetate NaOAc (65mg, 0.8mmol), PivOH pivalate (20mg, 0.2mmol), tetrabutylammonium bromide TBAB (32mg, 0.1mmol) and 2.5mL N,N-dimethylformaldehyde A mixed solvent of amide and dimethyl sulfoxide was stirred at 130°C for 24 hours. After cooling to room temperature, filter through diatomaceous earth, add 20 mL of water to the filtrate, extract the aqueous phase (2×15 mL) with dichloromethane, and separate the organic phase. The organic phases were mixed, dried over anhydrous magnesium sulfate, and filtered. The volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate / dichloromethane, v / v / v=250:8:20), The product 1a (53 mg, ...
Embodiment 2
[0031] Reaction steps and operation are with embodiment 1, and difference with embodiment 1 is that catalyst is palladium chloride PdCl 2 . The reaction was stopped, and the target product 1a (39 mg, yield 47%) was obtained after post-treatment. It shows that palladium chloride can also be used as a catalyst for the reaction, but it is not the best catalyst.
Embodiment 3
[0033] Reaction steps and operation are with embodiment 1, and difference with embodiment 1 is that catalyst is tetrakis triphenyl phosphopalladium Pd (PPh 3 ) 4 . The reaction was stopped, and the target product 1a (25 mg, yield 30%) was obtained after post-processing. It shows that tetrakistriphenylphosphopalladium can also be used as a catalyst for the reaction, but it is not the best catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com