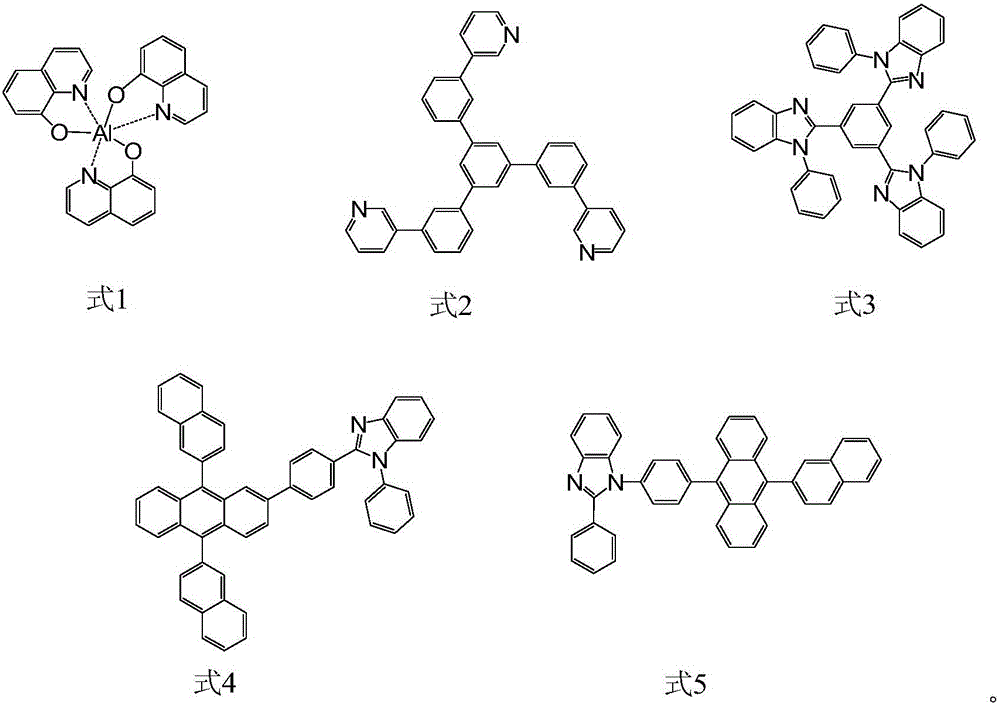
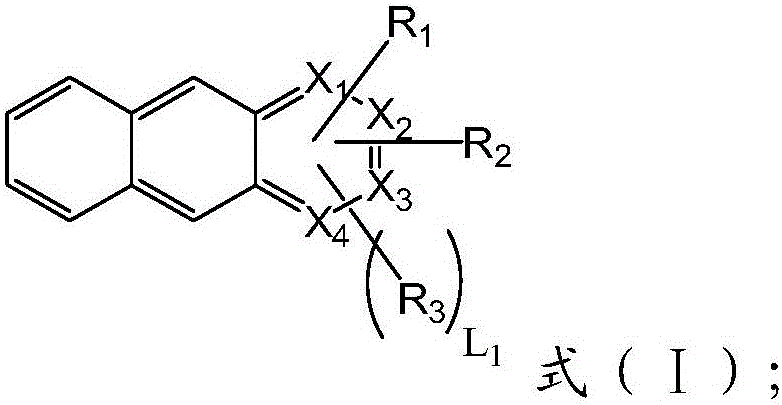
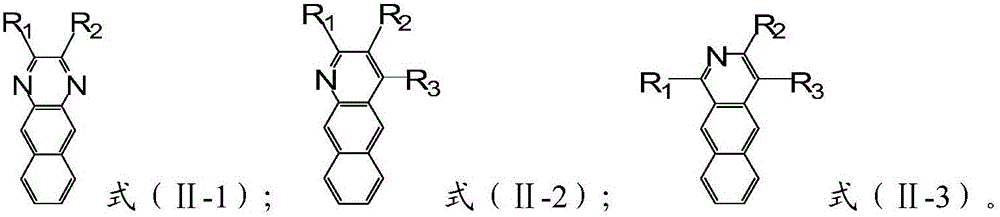Aromatic heterocyclic compound, preparation method thereof and organic electroluminescence device
A technology of compounds and aromatic heterocycles, applied in the field of organic optoelectronic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0113] The present invention also provides a method for preparing the above aromatic heterocyclic compound, comprising:
[0114] L 1 When it is 0, the compound shown in formula (III) is reacted with the compound shown in formula (IV) and the compound shown in formula (V) to obtain the aromatic heterocyclic compound shown in formula (I);
[0115] or L 1 When it is 1, the compound shown in the formula (III) is reacted with the compound shown in the formula (IV), the compound shown in the formula (V), and the compound shown in the formula (VI), to obtain the compound shown in the formula (I) aromatic heterocyclic compounds;
[0116] Y 1 -R 1 Formula (IV); Y 1 -R 2 Formula (V); Y 1 -R 3 Formula (Ⅵ);
[0117]
[0118] in,
[0119] x a 、X b 、X c 、X d Independently preferably -C-Br, -C-Cl or -N;
[0120] Y 1 Preferably -B(OH) 2 , boron compound group, -NH-R 1 or -NH 2 In the present invention, the boron compound group refers to the boron compound, the remainin...
Embodiment 1
[0156] Preparation of intermediate methyl 2-(6-chloropyrazine-2-carbonyl)benzoate (A-1)
[0157]
[0158] Dissolve 2-bromo-6-chloropyrazine (19.3g, 0.1mol) in 300mL of anhydrous ether, in a dry ice bath at -78°C, under nitrogen protection, add 44mL of BuLi (2.5M), stir the reaction for 1 hour, then add Dimethyl phthalate (19.4 g, 0.1 mol) was reacted for 2 hours, then gradually raised to room temperature, and water was added to terminate the reaction. Post-treatment process: the system is separated, the water layer is separated, the water layer is extracted once with ethyl acetate, the organic layer is combined and the organic solvent is spin-dried, and the column is separated with dichloromethane:petroleum ether=9:1 (volume ratio). A white solid (A-1) (16.6 g, y (yield) = 60%) was obtained.
Embodiment 2
[0160] Synthesis of Intermediates A-2~A-28:
[0161] According to the synthesis method of intermediate A-1 in Example 1 above, the compounds shown in Table 1 were prepared with the same molar ratio. Table 1 is a summary of the reaction substances, generated substances and yields of Example 2 of the present invention.
[0162] Table 1 Example 2 of the present invention reaction substance, generated substance and yield summary
[0163]
[0164]
[0165]
[0166]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com