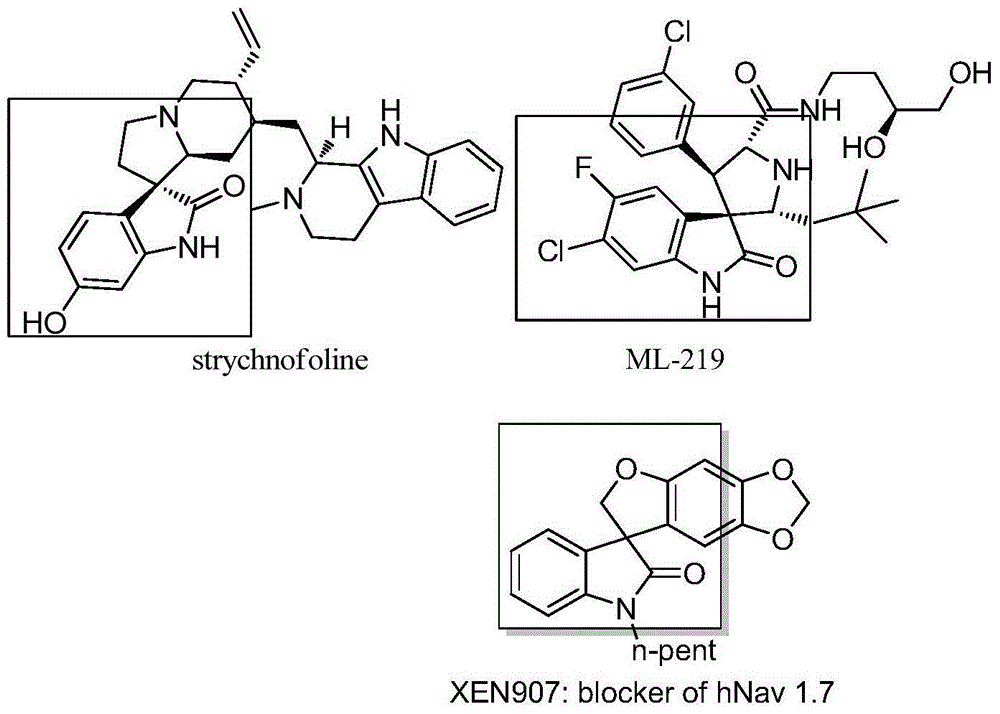
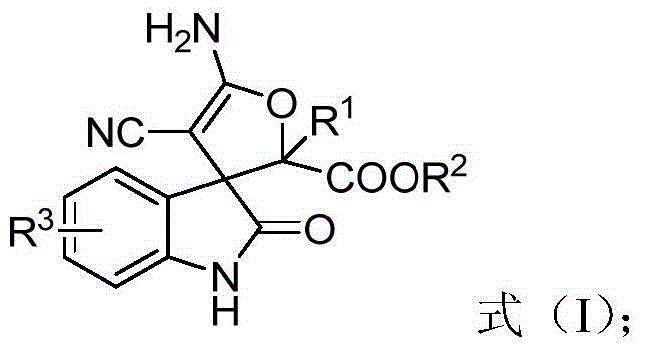
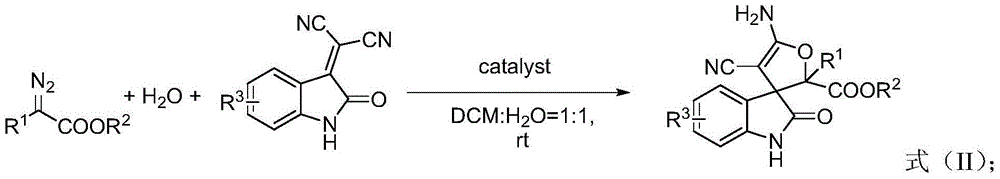
3,3'-dihydrofuran spiro-oxoindole derivative and preparation method and application thereof
A technology of dihydrofuran spiro and indole derivatives, which is applied in the fields of organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problems of decreased reaction selectivity, irritating odor, inconvenient experimental operation, etc., and achieves atomic utilization. High yield, high yield and wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Weigh 3-dicyanomethyleneoxindole (0.5 mmol) into a 50 mL eggplant-shaped bottle, and add 5 mL of commercially available reagent grade dichloromethane. Take another small bottle and add 5mL of water, weigh copper trifluoromethanesulfonate (0.05mmol), mix well, transfer to an eggplant-shaped bottle, and stir thoroughly. Weigh ethyl diazoacetate (2.0 mmol), add dichloromethane to make 4 mL mixed solution. The mixed solution was withdrawn with a 5 mL syringe, and injected into a mixed solution containing 3-dicyanomethyleneoxindole, copper trifluoromethanesulfonate, and dichloromethane through a peristaltic pump control. After stirring at room temperature for 1 hour, the layers were separated. The lower organic phase was collected and extracted three times with 15 mL of ethyl acetate. The upper organic phases were combined and dried over anhydrous sodium sulfate. After filtration, a1 and a2 were separated by column chromatography (eluent:petroleum ether:ethyl acetate with ...
Embodiment 2
[0042] Weigh 5-methyl-3-dicyanomethyleneoxindole (0.5 mmol) into a 50 mL eggplant-shaped bottle, and add 5 mL of commercially available reagent grade dichloromethane. Take another small bottle and add 5mL of water, weigh copper trifluoromethanesulfonate (0.05mmol), mix well, transfer to an eggplant-shaped bottle, and stir thoroughly. Weigh ethyl diazoacetate (2.0 mmol), add dichloromethane to make 4 mL mixed solution. The mixed solution was withdrawn with a 5 mL syringe, and injected into a mixed solution containing 5-methyl-3-biscyanomethyleneoxindole, copper trifluoromethanesulfonate, and dichloromethane through a peristaltic pump. After stirring at room temperature for 1 hour, the mixture was allowed to stand still to separate layers. The lower organic phase was collected and extracted three times with 15 mL of ethyl acetate. The upper organic phases were combined and dried over anhydrous sodium sulfate. After filtration, b1 and b2 were separated by column chromatography...
Embodiment 3
[0048] Weigh 5-methoxy-3-dicyanomethyleneoxindole (0.5 mmol) into a 50 mL eggplant-shaped bottle, and add 5 mL of commercially available reagent grade dichloromethane. Take another small bottle and add 5mL of water, weigh copper trifluoromethanesulfonate (0.05mmol), mix well, transfer to an eggplant-shaped bottle, and stir thoroughly. Weigh ethyl diazoacetate (2.0 mmol), add dichloromethane to make 4 mL mixed solution. The mixed solution was withdrawn with a 5 mL syringe, and injected into a mixed solution containing 5-methoxy-3-biscyanomethyleneoxindole, copper trifluoromethanesulfonate, and dichloromethane through a peristaltic pump. After stirring at room temperature for 1 hour, the mixture was allowed to stand still to separate layers. The lower organic phase was collected and extracted three times with 15 mL of ethyl acetate. The upper organic phases were combined and dried over anhydrous sodium sulfate. After filtration, c1 and c2 were separated by column chromatograp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com