Naphthalene acetamide compound
A technology for compounds and diseases, applied in the field of medicine, can solve problems such as unsatisfactory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
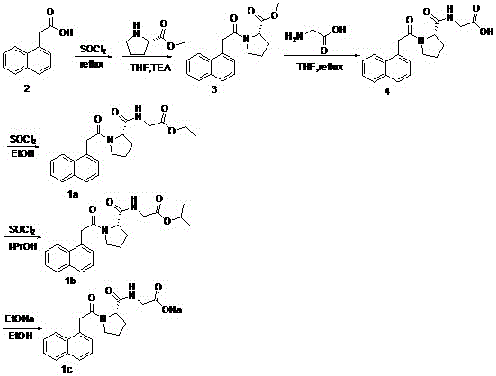
[0013] Embodiment 1: the preparation of each compound:
[0014] Because the compounds described herein have strong coherence, in order to describe the preparation method of each compound in detail, accurately and conveniently, it is expressed with 1 embodiment, and the serial number of the compound is indicated below each compound of the following synthetic route, for more Concise descriptions are replaced by serial numbers in the following preparation methods:
[0015]
[0016] (1) Synthesis of Compound 3:
[0017] Accurately weigh 93.1 grams of raw material 2 and add it to 200.0 mL of thionyl chloride, stir the reaction at 80°C for 6 hours, stop the reaction, cool the reaction solution to room temperature, add 75.7 grams of triethylamine and 300 mL of tetrahydrofuran, and stir for 20 minutes. Dissolve 1.0 gram of L-proline methyl ester in 200 mL of tetrahydrofuran, slowly add it dropwise to the above reaction solution, continue the reaction for 4 hours, stop the reaction...
Embodiment 2
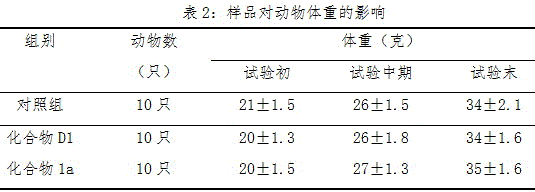
[0025]Example 2: Effects of Compound 1a and Compound D1 in Example 1 on Cerebral Infarction Volume in Rats with Focal Cerebral Ischemia
[0026] (1) Experimental materials and methods
[0027] Wistar rats, body weight 250-280g. They were reared separately before and after the operation, the room temperature was kept at 23-25°C, and they had their own food and water. The tMCAO model was prepared according to the method of Longa et al. Rats were anesthetized with 10% chloral hydrate (350mg / kg, i.p.), the body temperature was maintained at 37±0.5°C, and the supine position was fixed on the operating table. The skin was incised along the midline of the neck, and the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were carefully separated. Cut the ECA ligation and straighten it to be in line with the ICA. Cut a small opening on the ECA, and insert a 4.0cm-long, 0.26mm-diameter round siliconized nylon rope (coated with 0.1% pol...
Embodiment 3
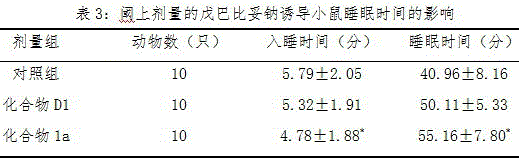
[0038] Example 3: Effects of compound 1a and compound D1 on sleep in rats:
[0039] sleep improvement test
[0040] Animal source: Kunming white mice, 18-22 grams, male. Experimental animal breeding room temperature 22 ± 2 ℃, relative humidity 50-70%. In this experiment, the dose of compound 1a was set at 25 mg / kg, and a distilled water control group was also set up.
[0041] Sample treatment: Take 25mg of each sample and add distilled water to 20ml to make a uniform suspension for testing.
[0042] Way of giving samples: gavage
[0043] Experimental method: pentobarbital sodium suprathreshold dose hypnosis test:
[0044] Select 30 male mice with a body weight of 18-22g, and randomly divide them into 3 groups, 10 in each group, give samples continuously for 30 days, and give each group of animals 50 mg / kg. Barbital sodium was injected intraperitoneally, and the injection volume was 0.2ml / 20g.b.w. The mouse's righting reflex disappeared for more than 1 minute as the criter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com