Macromolecule cross-linking agent based on functional cellulose ester and preparation method and application thereof
A technology of macromolecular crosslinking agent and cellulose ester, which is applied in the field of macromolecular crosslinking agent based on functional cellulose ester and its preparation, can solve the limitations of gelatin film tensile and biodegradation resistance, crosslinking Low degree, unfavorable problems, etc., to achieve the effect of improving mechanical properties and surface hydrophobicity, high resistance to degradation, and inhibiting in vitro degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Preparation of macromolecular crosslinking agent based on functional cellulose ester
[0064] (1) Synthesis of citric anhydride (CAD):
[0065] Weigh the corresponding mass of anhydrous citric acid (CA), acetic acid and acetic anhydride in a molar ratio of 1:2:1.2, and react at 35°C for 18h. After the reaction was over, the solvent acetic acid was distilled off under reduced pressure, and the residual oily product was added with 3 times the volume of chloroform of the reaction solution while stirring, and a large amount of white solids were precipitated. Suction filter and wash with chloroform, dry to obtain citric anhydride (CAD).
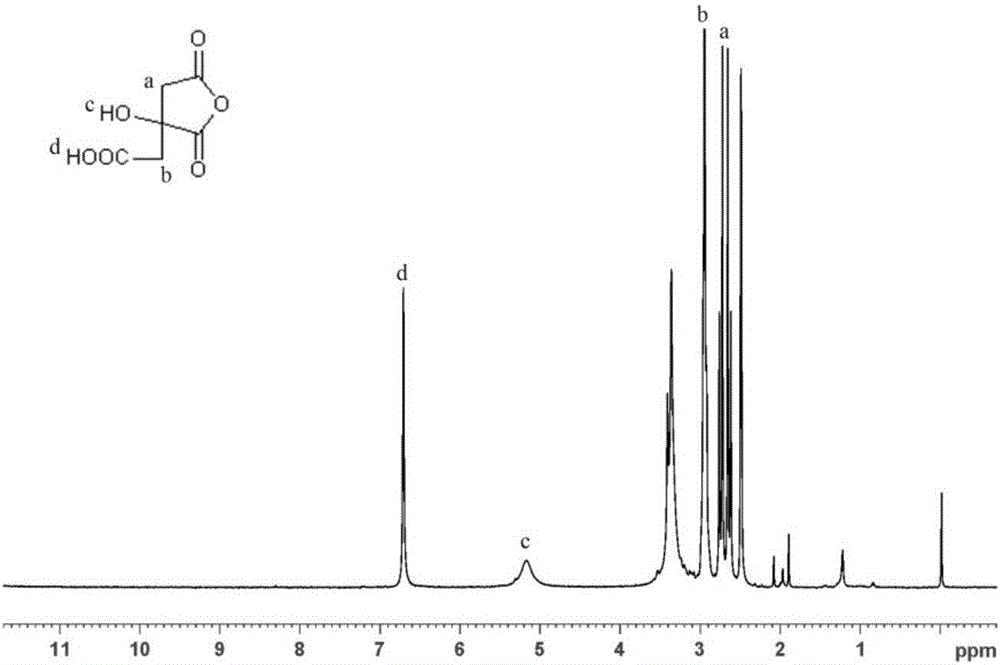
[0066] like figure 1 as shown, 1 HNMR (400MHz, DMSO): δ2.761, 2.618(d, 2H), δ2.945, 2.921(d, 2H), δ5.174(s, 1H), δ6.706(s, 1H), δ3.080 (s, DMSO), δ2.496-2.488 (m, DMSO);
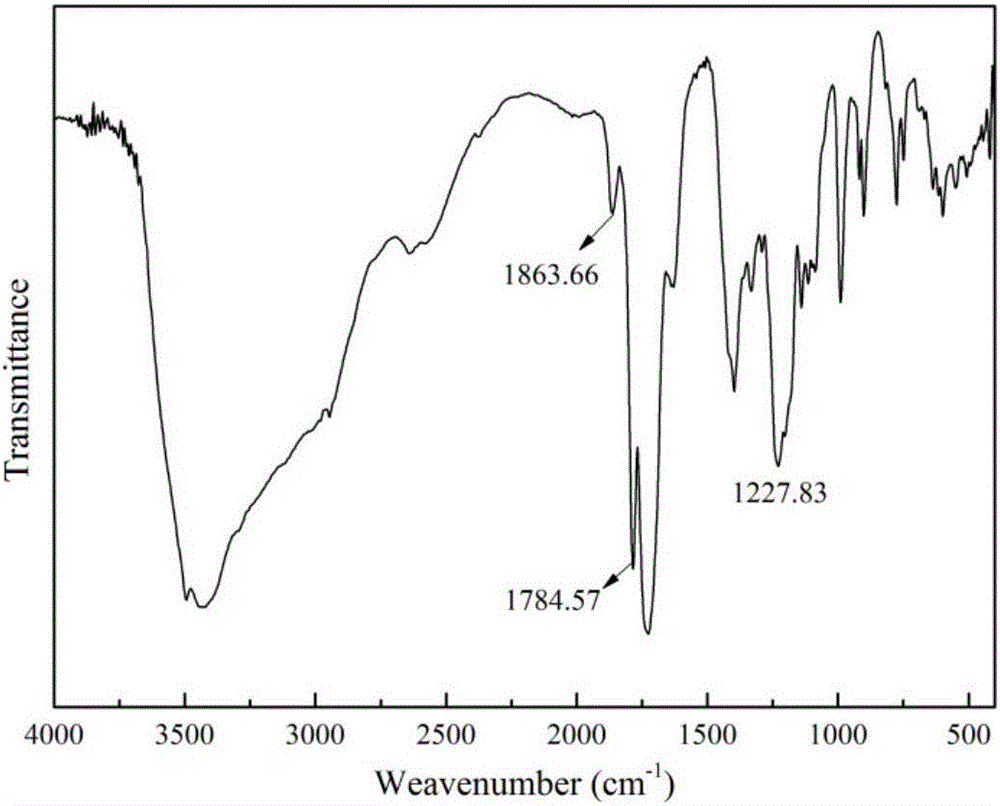
[0067] like figure 2 Shown, FT-IR: at 1863.66cm -1 and 1784.57cm -1 The characteristic absorption peak of acid anhydride appears at 1784.57cm -1 The ...
Embodiment 2
[0086] Example 2: Preparation of macromolecular crosslinking agent based on functional cellulose ester
[0087] (1) Synthesis of citric anhydride (CAD):
[0088] Weigh corresponding masses of anhydrous citric acid (CA), acetic acid and acetic anhydride in a molar ratio of 1:2:1.5, and react at 37°C for 18h. After the reaction was over, the solvent acetic acid was distilled off under reduced pressure, and chloroform of 4 times the volume of the reaction solution was added to the residual oil under stirring conditions, and a large amount of white solids were precipitated. Suction filter and wash with chloroform, dry to obtain citric anhydride (CAD).
[0089] (2) Preparation of CAD functionalized microcrystalline cellulose (MC):
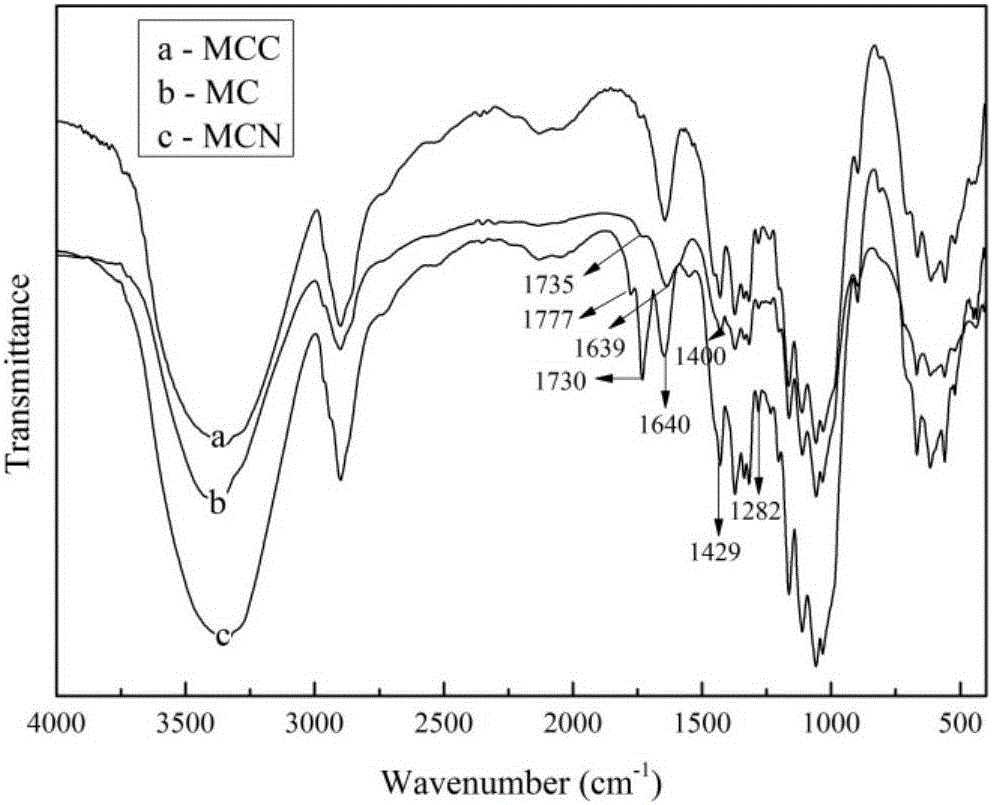
[0090] Weigh 1 g of microcrystalline cellulose (MCC) and 5 g of CAD in 40 ml of DMF, stir magnetically in an oil bath at 65 °C for 20 h, wash with 10 ml of DMF, 100 ml of distilled water, 30 ml of saturated NaHCO3 solution, 200 ml of distilled water, an...
Embodiment 3
[0093] Embodiment 3: Preparation of macromolecular crosslinking agent based on functional cellulose ester
[0094] (1) Synthesis of citric anhydride (CAD):
[0095] Weigh corresponding masses of anhydrous citric acid (CA), acetic acid and acetic anhydride at a molar ratio of 1:2:1.8, and react at 40°C for 16h. After the reaction, the solvent acetic acid was distilled off under reduced pressure, and the residual oily product was added with 5 times the volume of the reaction solution of chloroform under stirring conditions, and a large amount of white solids were precipitated. Suction filter and wash with chloroform, dry to obtain citric anhydride (CAD).
[0096] (2) Preparation of CAD functionalized microcrystalline cellulose (MC):
[0097] Weigh 1g of microcrystalline cellulose (MCC) and 8g of CAD in 60ml of DMF, stir magnetically in an oil bath at 72°C for 22 hours, wash with 15ml of DMF, 150ml of distilled water, 40ml of saturated NaHCO3 solution, 300ml of distilled water,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com