Preparation method of spherical lithium nickel manganese oxide material with hollow porous micro-nano level structure
A micro-nano structure, lithium nickel manganese oxide technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of limited wide application, poor repeatability, low product volume, etc., to improve cycle performance, good rate performance and Cycle performance, effect of increasing contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
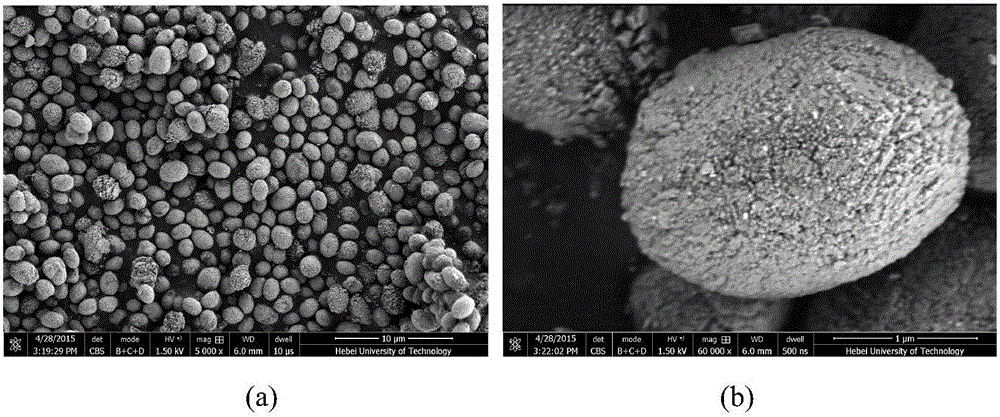
[0032] Weigh 0.9954g (0.004mol) nickel acetate (Ni(CH 3 COO) 2 4H 2 O), 2.9411g (0.012mol) manganese acetate (Mn(CH 3 COO) 2 4H 2 (0) and 1.9219g (0.032mol) urea were dissolved in the mixed solution of 80mL deionized water and ethylene glycol (volume ratio 5:1), and magnetic stirring made it dissolve completely for 30 minutes; In a 100mL high-pressure reactor lined with vinyl fluoride, seal it and place it in an oven for 8 hours at 170°C for 8 hours, then cool it down to room temperature naturally, wash, filter, and dry the resulting precipitate to obtain Ni 0.25 mn 0.75 CO 3 precursor, its SEM as figure 1 shown. Depend on figure 1 (a) It can be seen that the prepared precursors are all spherical particles with a uniform particle size, which is basically maintained at 1-2 μm; figure 1 (b) It can be seen that the surface of the precursor is denser.
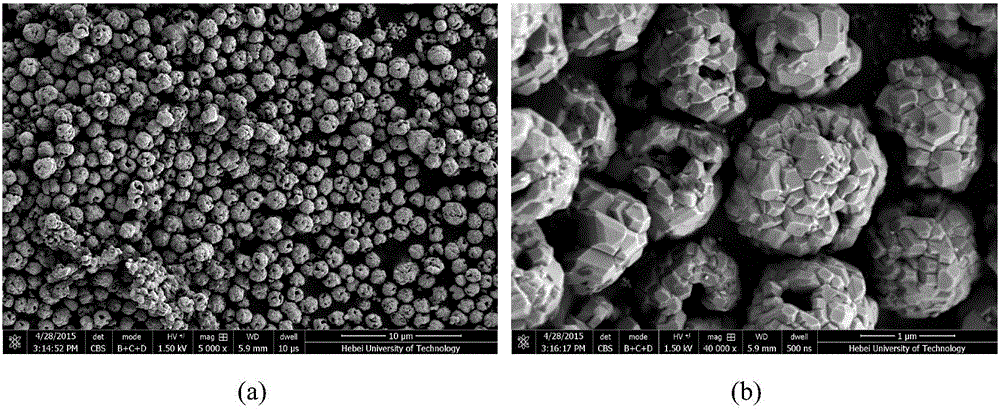
[0033] The obtained precursor was pre-calcined in air at 500°C for 4 hours to obtain the oxide, and its SEM was as fol...
Embodiment 2
[0036] Weigh 0.4977g (0.002mol) nickel acetate (Ni(CH 3 COO) 2 4H 2 O), 1.4705g (0.006mol) manganese acetate (Mn(CH 3 COO) 2 4H 2 (0) and 0.8408g (0.014mol) urea were dissolved in the mixed solution of 80mL deionized water and ethylene glycol (volume ratio 3:1), and magnetic stirring made it dissolve completely for 30 minutes; In a 100mL high-pressure reactor lined with vinyl fluoride, seal it and place it in an oven at 180°C for 6 hours, then cool it down to room temperature naturally, wash, filter, and dry the resulting precipitate to obtain Ni 0.25 mn 0.75 CO 3 Precursor,
[0037] Pre-burn the obtained precursor in the air at 500°C for 4 hours to obtain the oxide, put it into absolute ethanol (the volume is 15 times the volume of the oxide), and then press Li:(Ni+Mn)=1.05: 2 (molar ratio) weigh 0.1552gLi 2 CO 3 Add it, put the mixed solution on a magnetic stirrer at 80°C while heating and stirring until the ethanol is completely volatilized, calcinate the obtained...
Embodiment 3
[0039] Weigh 0.7466g (0.003mol) nickel acetate (Ni(CH 3 COO) 2 4H 2 O), 2.2058g (0.009mol) manganese acetate (Mn(CH 3 COO) 2 4H 2 (0) and 1.6216g (0.027mol) urea were dissolved in the mixed solution of 80mL deionized water and ethylene glycol (volume ratio 2:1), and magnetic stirring made it dissolve completely for 30 minutes; In a 100mL high-pressure reactor lined with vinyl fluoride, seal it and place it in an oven for 4 hours at 190°C to react for 4 hours, then cool to room temperature naturally, wash, filter, and dry the resulting precipitate to obtain Ni 0.25 mn 0.75 CO 3 Precursor,
[0040] Pre-burn the obtained precursor in the air at 500°C for 4 hours to obtain the oxide, put it into absolute ethanol (the volume is 10 times the volume of the oxide), and then press Li:(Ni+Mn)=1.06: 2 (molar ratio) weigh 0.2350gLi 2 CO 3 Add it, put the mixed solution on a magnetic stirrer at 80°C while heating and stirring until the ethanol is completely volatilized, calcinate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com